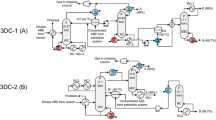
Abstract
This paper presents an analysis of the literature on the complex study of the reaction and separation constituents of technologies for obtaining industrial organic products: cyclohexanone, epichlorohydride, isopropylbenzene, glycols, and methyl isobutyl ketone. Ways that make it possible to reduce energy consumption by replacing solvents or changing the reaction conditions and combining different design variants of both blocks are shown. The dependence of the energy consumption of the separation flowsheet on the operating conditions of the reactor unit is shown using the example of a mixture of methyl isobutyl ketone. The structure of the diagram of an eight-component system is studied and thermodynamic restrictions on the distillation process are determined. The distillation process is calculated (for a separation flowsheet of a fixed structure) for four compositions of the initial mixture (obtained under different operating conditions of the reactor), and the parameters of the column work making it possible to obtain commercial quality products and corresponding to minimal energy consumption are determined.

Similar content being viewed by others
REFERENCES
Timofeev, V.S., Serafimov, L.A., and Timoshenko, A.V., Printsipy tekhnologii osnovnogo organicheskogo i neftekhimicheskogo sinteza: uchebnoe posobie dlya vuzov (Principles of the Technology of Basic Organic and Petrochemical Synthesis: A Textbook for Institutions of Higher Education), Moscow: Vysshaya Shkola, 2010.
Pavlov, S.Yu., Kulov, N.N., and Kerimov, R.M., Improvement of chemical engineering processes using systems analysis, Theor. Found. Chem. Eng., 2014, vol. 48, no. 2, pp. 117–126. https://doi.org/10.1134/S0040579514020109
Serafimov, L.A., Timofeev, V.S., and Frolkova, A.K., Qualitative investigations of production processes as a stage of their intensification based on mathematical modeling using a computer, Intensif. Tekhnol. Protsessov: Mater., Tekhnol., Oborud., 2009, no. 6, p. 9.
L’vov, S.V., Nekotorye voprosy rektifikatsii binarnykh i mnogokomponentnykh smesei (Some Problems of the Distillation of Binary and Multicomponent Mixtures), Moscow: Akad. Nauk SSSR, 1960.
Opredelyaya strategii khimicheskoi otrasli. K 60-letiyu OAO “NIITEKhIM” (Determining the Strategies of the Chemical Industry: The 60th Anniversary of OAO NIITEKhIM), Moscow: NIITEKhIM, 2018.
Kulov, N.N., Engineering problems of increasing energy efficiency of chemical technological processes and apparatus, Sbornik plenarnykh dokladov Mezhdunarodnogo nauchno-tekhnicheskogo foruma “Pervye mezhdunarodnye Kosyginskie chteniya “Sovremennye zadachi inzhenernykh nauk” (International Scientific and Technical Forum “First International Kosygin Readings: Current Topics in Engineering Sciences”: A Collection of Plenary Lectures), Moscow: Ross. Gos. Univ. im. A.N. Kosygina, 2017, vol. 1, pp. 26–32.
Frolkova, A.K., Maevskii, M.A., Oshanina, I.V., and Frolkova, A.V., Cyclohexanone: The main methods of obtaining and extracting target products from a reaction mixture, Theor. Found. Chem. Eng., 2018, vol. 52, no. 4, pp. 653–660. https://doi.org/10.1134/S0040579518040097
Temkin, O.N., Bruk, L.G., Zakharova, D.S., Odintsov, K.Yu., Katsman, E.A., Petrov, I.V., and Istomina, O.Yu., Kinetics of cyclohexene oxidation by p-quinones in aqueous-organic solutions of cationic palladium(II) complexes, Kinet. Catal., 2010, vol. 51, no. 5, pp. 691–703. https://doi.org/10.1134/S0023158410050101
Temkin, O.N., Frolkova, A.V., Zakharova, D.S., Bruk, L.G., et al., Effect of the composition of the CH3CN–H2O binary solvent on the kinetics of the oxidation of cyclohexene with p-benzoquinone in solutions of the cationic complexes of palladium(II), Tonkie Khim. Tekhnol., 2015, vol. 10, no. 3, pp. 77–84.
Martynov, I.V., Efremov, G.E., and Bovyrina, E.A., The mechanism and kinetic models of the catalytic oxidation of ethylene by p-benzoquinone in aqueous-acetonitrile solutions of Pd(II) cationic complexes, Kinet. Catal., 2018, vol. 59, p. 436.
Efremov, G.E., Bovyrina, E.A., Podtyagina, A.V., Oshanina, I.V., and Temkin, O.N., Ethylene and cyclohexene oxidation by p-benzoquinone, hydrogen peroxide, and oxygen in the solutions of cationic Pd(II) complexes in acetonitrile–water and ionic liquid–water binary solvents, Kinet. Catal., 2019, vol. 60, p. 52.
Frolkova, A.V., Balbenov, S.A., Frolkova, A.K., and Akishina, A.A., Phase equilibrium in the system water–acetonitrile–cyclohexene–cyclohexanone, Russ. Chem. Bull., 2015, vol. 64, no. 10, pp. 2330–2336. https://doi.org/10.1007/s11172-015-1160-7
Frolkova, A., Zakharova, D., Frolkova, A., and Balbenov, S., Liquid–liquid and liquid–liquid equilibrium for ternary system water–acetonitrile–cyclohexene at 298.15 K, Fluid Phase Equilib., 2016, vol. 408, pp. 10–14.
Frolkova, A.V., Mayevskiy, M.A., and Frolkova, A.K., Analysis of phase equilibrium diagrams of cyclohexene + water + cyclohexanone + solvent system, J. Chem. Eng. Data, 2018, vol. 63, no. 3, p. 679.
Frolkova, A.V., Mayevskiy, M.A., and Smirnov, A.Y., Phase equilibrium of systems cyclohexene + water + cyclohexanone + N-methyl-2-pyrrolidone (+acetonitrile), J. Chem. Eng. Data, 2019, vol. 64, no. 6, p. 2888.
Zhuchkov, V., Frolkova, A., and Nazanskiy, S., Experimental measurements of mutual solubility of cyclohexanone, cyclohexene and ionic liquid, Chem. Pap., 2018, vol. 72, p. 1107.
Sulimov, A.V., Danov, S.M., Ovcharova, A.V., Obryvalina, A.N., Telyashev, R.G., Ovcharov, A.A., and Balashov, A.L., Innovative processes for producing epichlorohydrin, Neftegazokhimiya, 2013, no. 1, p. 32.
Danov, S.M., Sulimov, A.V., and Sulimova, A.V., Solvent effect on epoxidation of allyl chloride with hydrogen peroxide on titanium-containing silicalite, Russ. J. Appl. Chem., 2008, vol. 81, no. 11, pp. 1963–1966. https://doi.org/10.1134/S1070427208110189
Ovcharova, A.V., Development of a technology for epichlorohydrin production, Extended Abstract of Cand. Sci. (Chem.) Dissertation, Moscow: Mendeleev Univ. of Chemical Technology of Russia, 2012.
Frolkova, A.V., Okhlopkova, E.A., and Frolkova, A.K., Phase equilibrium of reaction mixtures for the production of epichlorohydrin in the presence of solvents, Theor. Found. Chem. Eng., 2019, vol. 53, no. 2, pp. 185–192. https://doi.org/10.1134/S0040579519020039
Okhlopkova, E.A. and Frolkova, A.V., Comparative analysis of separation schemes of reaction mixtures of epichlorohydrin production in the presence of various solvents, Theor. Found. Chem. Eng., 2019, vol. 53, no. 6, p. 1028.
Serafimov, L.A., Thermodynamic and topological analysis of heterogeneous equilibrium diagrams of multicomponent mixtures, Russ. J. Phys. Chem. A, 2002, vol. 76, no. 8, p. 1211.
Serafimov, L.A., State of the art in the thermodynamic and topological analysis of phase diagrams, Theor. Found. Chem. Eng., 2009, vol. 43, no. 3, pp. 268–278. https://doi.org/10.1134/S0040579509030051
Latyipov, R.M., Osipov, E.V., and Telyakov, E.Sh., Systems analysis of equipment and technology for glycols production, Theor. Found. Chem. Eng., 2017, vol. 51, no. 6, pp. 980–991. https://doi.org/10.1134/S0040579517060112
Chudinova, A.A., Nurmakanova, A.E., Salishcheva, A.A., Ivashkina, E.N., and Gavrikov, A.A., Effect of the operating parameters of an alkylation reactor on the concentration of n-propylbenzene in the product mixture, Khim. Interesakh Ustoich. Razvit., 2014, no. 22, p. 569.
Chudinova, A.A., Optimization of the operation of a reactor and a distillation unit for separating the products of liquid-phase alkylation of benzene with propylene for enhancing the quality and yield of isopropyl benzene, Extended Abstract of Cand. Sci. (Eng.) Dissertation, Tomsk: National Research Tomsk Polytechnic Univ., 2016.
Al-Rabiah, A.A., Bagabas, A.A., and Akhmedov, V.M., EP Patent 2532642, 2017.
Zharov, V.T. and Serafimov, L.A., Fiziko-khimicheskie osnovy distillyatsii i rektifikatsii (Physicochemical Fundamentals of Distillation Processes), Leningrad: Khimiya, 1975.
Liu, D., Li, L., Lv, R., and Chen, Y., Liquid–liquid equilibria for the ternary system mesityl oxide + phenol + water at 298.15, 313.15, and 323.15 K, J. Chem. Eng. Data, 2016, vol. 61, no. 7, p. 2493.
Rawat, B.S. and Krlshna, S., Isobaric vapor-liquid equilibria for the partially miscible system of water-methyl isobutyl ketone, J. Chem. Eng. Data, 1984, vol. 29, no. 4, p. 403.
Ogorodnikov, S.K., Lesteva, T.M., and Kogan, V.B., Azeotropnye smesi. Spravochnik (Azeotropic Mixtures: A Handbook), Leningrad: Khimiya, 1971.
Tochigi, K., Inoue, H., and Kojima, K., Determination of azeotropes in binary systems at reduced pressures, Fluid Phase Equilib., 1985, vol. 22, p. 343.
Jungho, C. and Jong-Ki, J., Optimization study on the azeotropic distillation process for isopropyl alcohol dehydration, Korean J. Chem. Eng., 2006, vol. 23, no. 1, p. 1.
Tae-hwan, C., Kenji, O., and Kazuo, K., Measurement of vapor-liquid equilibrium for systems with limited miscibility, Fluid Phase Equilib., 1983, vol. 11, p. 137.
Feng, Y., Song, H., Xiao, M., Lin, K., Guo, K., and Gai, H., Development of phenols recovery process from coal gasification wastewater with mesityl oxide as a novel extractant, J. Cleaner Prod., 2017, vol. 166, p. 1314.
Hack, C.W. and Van Winkle, M., Vapor-liquid equilibria of the diacetone alcohol-water system at subatmospheric pressures, Ind. Eng. Chem., 1954, vol. 46, no. 11, p. 2392.
Yakhyaev, M.A., Gutenkov, V.S., Cardona, C.A., and Pisarenko, Yu.A., Development of a reactive distillation process for producing mesityl oxide. II. Analysis of statics and modeling of the process, Tonkie Khim. Tekhnol., 2019, vol. 14, no. 2, p. 23.
Yang, C., Qian, Y., Guo, J., Chen, J., and Peng, J., Liquid–liquid equilibria for the ternary system methyl isobutyl ketone + m-benzenediol + water, J. Chem. Eng. Data, 2014, vol. 59, p. 3324.
Yang, C., Qian, Y., Jiang, Y., and Zhang, L., Liquid–liquid equilibria for the quaternary system methyl isobutyl ketone–water–phenol–hydroquinone, Fluid Phase Equilib., 2007, vol. 258, p. 73.
Tan, Y., Guo, C., Qian, Y., and Yang, S., Liquid–liquid equilibrium for ternary systems of 2-pentanone/mesityl oxide + catechol + water at 298.2, 318.2, and 333.2 K, J. Chem. Eng. Data, 2018, vol. 63, no. 8, p. 3117.
Chen, H. and Wagner, J., Mutual solubilities of alkylbenzene + water systems at temperatures from 303 to 373 K: Ethylbenzene, p-xylene, 1,3,5-trimethylbenzene, and butylbenzene, J. Chem. Eng. Data, 2018, vol. 39, p. 679.
Ozmen, D., (Liquid + liquid) equilibria of (water + propionic acid + methyl isoamyl ketone or diisobutyl ketone or ethyl isoamyl ketone) at T = 298.2 K, Fluid Phase Equilib., 2006, vol. 250, p. 70.
Serafimov, L. and Frolkova, A., Determination of vapor–liquid equilibrium diagrams of multicomponent systems, Chem. Pap., 2016, vol. 70, p. 1578.
Funding
The work was supported by the Ministry of Education and Science of the Russian Federation as part of State Assignment no. 0706-2020-0020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Muravev
Rights and permissions
About this article
Cite this article
Frolkova, A.V., Mayevskii, M.A., Frolkova, A.K. et al. Developing Energy-Efficient Technologies for Obtaining Organic Substances Based on a Comprehensive Study of the Reaction and Separation Constituents. Theor Found Chem Eng 54, 1215–1222 (2020). https://doi.org/10.1134/S0040579520060159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520060159