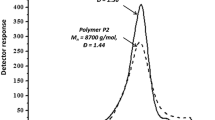
Abstract
Donor–acceptor (D-A) type of new constituted diketopyrrolopyrrole-based polymers, PDPP-EDOT (poly A), PDPP-TP (poly B), and PDPP-EBTE (poly C), were synthesized by one-pot direct C–H heteroarylation polymerization methodology supported by Pd(OAc)2/Bu4NBr catalytic system. C–H heteroarylation coupling of highly selective sites avoided the difficulty of the synthesis using organometallic reagent and gave highly pure polymers in good yields. The targeted polymers were evaluated using NMR, TGA, DSC, UV–VIS absorption, GPC, and electrochemical cyclic voltammetry. The polymers are soluble in most of organic solvents, and TGA studies show their high thermally stability. Polymers bandgaps are tuned by alternative copolymerizing with different electron-donating and/or electron-withdrawing units. UV–VIS absorption spectra demonstrated that the polymers show excellent light absorption, with narrow optical bandgaps, around 1.20 eV, confirmed by cyclic voltammetry parameters. All of these promising properties indicated that they might be excellent polymeric materials for polymer solar cells, organic field effect transistors, and so on.





Similar content being viewed by others
References
Li Z, Chueh CC, Jen AKY (2019) Recent advances in molecular design of functional conjugated polymers for high-performance polymer solar cells. Prog Polym Sci 99:101175–101234
Cui C, Li Y (2019) High-performance conjugated polymer donor materials for polymer solar cells with narrow-bandgap nonfullerene acceptors. Energy Environ Sci 12:3225–3246
Günes S, Neugebauer H, Sariciftci NS (2007) Conjugated Polymer-Based Organic Solar Cells. Chem Rev 107(4):1324–1338
Kim M, Ryu SU, Park SA, Choi K, Kim T, Chung D, Park T (2019) Donor-acceptor conjugated polymer for high-performance organic field-effect transistors: a progress report. Adv Funct Mater 30(20):1904545–1904569
Allard S, Forster M, Souharce B, Thiem H, Scherf U (2008) Organic semiconductors for solutionprocessable field-effect transistors (OFETs). Angew Chem Int Ed 47:4070–4098
Giraud L, Grelier S, Grau E, Hadziioannou G, Brochon C, Cramail H, Cloutet E (2020) Upgrading the chemistry of p-conjugated polymers toward more sustainable materials. J Mater Chem C 8:9792–9810
Matsui H, Takeda Y, Tokito S (2019) Flexible and printed organic transistors: from materials to integrated circuits. Org Electron 75:105432–105449
Bao WW, Li R, Dai ZC, Tang J, Shi X, Geng JT, Deng ZF, Hua J (2020) Diketopyrrolopyrrole (DPP)-based materials and its applications: a review. Front Chem 8:679–684
Zhao C, Guo Y, Zhang Y, Yan N, You S, Li W (2019) Diketopyrrolopyrrole-based conjugated materials for non-fullerene organic solar cells. J Mater Chem A 7:10174–10199
Li W, Hendriks KH, Wienk MM, Janssen RAJ (2016) Diketopyrrolopyrrole polymers for organic solar cells. Acc Chem Res 49(1):78–85
Choi SH, Kwon OT, Kim NR, Yoon C, Kim JP, Choi JH (2010) Preparation of solvent soluble dyes derived from diketo-pyrrolo-pyrrole pigment by introducing an N-Alkyl group. Bull Kor Chem Soc 4:1073–1076
Sonar P, Ng GM, Lin TT, Dodabalapur A, Chen ZK (2010) Solution processable low bandgap diketopyrrolopyrrole (DPP) based derivatives: novel acceptors for organic solar cells. J Mater Chem 20(18):3626–3636
Liu Q, Bottle SE, Sonar P (2019) Developments of diketopyrrolopyrrole-dye-based organic semiconductors for a wide range of applications in electronics. Adv Mater 32(4):1903882–1903927
Keshtov ML, Kuklin SA, Konstantinov IO, Ostapov IE, Xie Zh, Koukaras EN, Suthar R, Sharma GD (2020) New Donor-Acceptor polymers with a wide absorption range for photovoltaic applications. Sol Energy 205:211–220
Kim JH, Kim KH, Shin J, Lee TW, Cho MJ, Choi DH (2013) Electrical and photoelectrical properties of polymer single nanowire made of diketopyrrolopyrrole-based conjugated copolymer bearing dithieno[3,2-b:2′,3′-d]thiophene. Synth Met 167:37–42
Alqurashy BA (2019) Preparation and physical characterization of pyrene and pyrrolo[3,4-c]pyrrole-1,4-dione-based copolymers. Chem Open 8(4):429–433
Morin PO, Bura T, Leclerc M (2016) Realizing the full potential of conjugated polymers: innovation in polymer synthesis. Mater Horiz 3(1):11–20
Cheng YJ, Yang SH, Hsu CS (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109(11):5868–5923
Owczarczyk ZR, Braunecker WA, Garcia A, Larsen R, Nardes AM, Kopidakis N, Olson DC (2013) 5,10-dihydroindolo[3,2-b]indole-based copolymers with alternating donor and acceptor moieties for organic photovoltaics. Macromolecul 46(4):1350–1360
Guzmán-Rabadán KK, Güizado-Rodríguez M, Barba V, Rodríguez M, Velusamy J, Ramos-Ortiz G (2020) Synthesis of fluorene-thiophene-benzothiadiazole (D-π-A) molecules by direct arylation reactions: formation of nanoparticles and their fluorescence study by one- and two-photon absorption. Opt Mater 101:109758–109769
Pankow R, Thompson B (2020) Approaches for Improving the sustainability of conjugated polymer synthesis using direct arylation polymerization (DArP). Polym Chem 11:630–640
Shaker M, Elhendawy M, Park B, Lee K (2019) New Designed isoindigo/thiophene medium-sized molecule containing π (D-A-D) Bridge with unexpected organic photovoltaic performance. New J Chem 43:18126–18133
Shaker M, Trinh CK, Kim W, Kim H, Lee K, Lee JS (2015) Direct C-H arylation synthesis of (DD′AD′DA′)-constituted alternating polymers with low bandgaps and their photovoltaic performance. New J Chem 39(6):4957–4964
Shaker M, Lee JH, Park B, Lee S, Lee K, Lee JS (2020) Synthesis and characterization of [A(DA`nD`)2] based small molecules with potential optoelectronic application. Synth Met 261:116307–116314
Shaker M, Lee JH, Trinh CK, Kim W, Kim H, Lee K, Lee JS (2015) A Facile method to synthesize strong push-pull [A`(D`AD)2]-based small molecules and their photovoltaic performance. RSC Adv 5:66005–66012
Stas S, Sergeyev S, Geerts Y (2010) Synthesis of diketopyrrolopyrrole (DPP) derivatives comprising bithiophene moieties. Tetrahedron 66(10):1837–1845
Shaker M, Park B, Lee JH, Kim W, Trinh CK, Lee HJ, Lee K, Lee JS (2017) Synthesis and organic field effect transistor properties of isoindigo/DPP-based polymers containing a thermolabile group. RSC Adv 7(27):16302–16310
Pilgram K, Zupan M, Skiles R (1970) Bromination of 2,1,3-benzothiadiazoles. J Heterocycl Chem 7(3):629–633
Hou Q, Zhou Q, Zhang Y, Yang W, Yang R, Cao Y (2004) Synthesis and electroluminescent properties of high-efficiency saturated red emitter based on copolymers from fluorene and 4,7-di(4-hexylthien-2-yl)-2,1,3-benzothiadiazole. Macromolecul 37(17):6299–6305
Kondratenko M, Moiseev AG, Perepichka DF (2011) New stable donor–acceptor dyads for molecular electronics. J Mater Chem 21(5):1470–1478
Zhou E, Cong J, Yamakawa S, Wei Q, Nakamura M, Tajima K, Yang C, Hashimoto K (2010) Synthesis of thieno[3,4-b]pyrazine-based and 2,1,3-benzothiadiazole-based donor-acceptor copolymers and their application in photovoltaic devices. Macromolecul 43(6):2873–2879
McNamara LE, Liyanage N, Peddapuram A, Murphy JS, Delcamp JH, Hammer NI (2016) Donor−acceptor−donor thienopyrazines via Pd-Catalyzed C−H activation as NIR fluorescent materials. J Org Chem 81:32–42
Zhao H, Liu C-Y, Luo S-C, Zhu B, Wang T-H, Hsu H-F, Yu H-H (2012) Facile syntheses of dioxythiophene-based conjugated polymers by direct C-H arylation. Macromolecul 45(19):7783–7790
Kuwabara J, Yamazaki K, Yamagata T, Tsuchida W, Kanbara T (2015) The effect of a solvent on direct arylation polycondensation of substituted thiophenes. Polym Chem 6(6):891–895
Leclerc M, Berrouard P (2013) Preparation of high molecular weight polymers 8y direct arylation and heteroarylation. Patent WO 2013056355:A1
Vezie M, Few S, Meager I et al (2016) Exploring the origin of high optical absorption in conjugated polymers. Nature Mater 15:746–753
Konkol KL, Schwiderski RL, Rasmussen SC (2016) Synthesis, characterization, and electropolymerization of extended fused-ring thieno[3,4-b]pyrazine-based terthienyls. Materials 9:404–419
Zoombelt AP, Gilot J, Wienk MM, Janssen RAJ (2009) Effect of extended thiophene segments in small band gap polymers with thienopyrazine. Chem Mater 21(8):1663–1669
Szumilo MM, Gann EH, McNeill CR, Lemaur V, Oliver Y, Thomsen L, Vaynzof Y, Sommer M, Sirringhaus H (2014) Structure influence on charge transport in naphthalenediimide–thiophene copolymers. Chem Mater 26:6796–6804
Koizumi Y, Ide M, Saeki A, Vijayakumar C, Balan B, Kawamoto M, Seki S (2013) Thienoisoindigo-based low-band gap polymers for organic electronic devices. Polym Chem 4:484–494
Misra A, Kumar P, Srivastava R, Dhawan SK, Kamalasanan MN, Chandra S (2005) Electrochemical and optical studies of conjugated polymers for three primary colours. IJPAP 43:921–925
Acknowledgements
This research was supported by the Egyptian Japanese Educational Partnership (EJEP) Fellowship, 2019. The author wishes to thank the Laboratory for Photofunctional Organic Chemistry at Nara Institute of Science and Technology for the assistance to complete this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaker, M. Comparative study of the optoelectronic properties of diketopyrrolopyrrole based polymers obtained by direct C-H arylation. Polym. Bull. 79, 829–842 (2022). https://doi.org/10.1007/s00289-021-03539-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03539-7