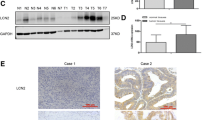
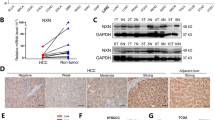
Abstract
LCN2 (Lipocalins) was first identified as iron transporter through associating with its siderophores and also involved in many cancer metastases, but its function is still paradoxical. We questioned that whether LCN2 might also associate exogenous iron chelator as does in inherent way and the association may influence their respective function. To address this issue, we investigated the effect of LCN2 on action of DpdtC (2,2′-dipyridine ketone hydrazone dithiocarbamte), an iron chelator in proliferation and metastasis-related gene expression. The results showed that exogenous LCN2 and DpdtC could inhibit growth of HepG2 cells, while the combination treatment enhanced their inhibitory effect both in proliferation and colony formation. This encouraged us to investigate the effect of the interaction on metastasis-related gene expression. The results revealed that both LCN2 and DpdtC impaired the wound healing of HepG2, but the inhibitory effect of DpdtC was significantly enhanced upon association with LCN2. Undergoing epithelium-mesenchymal transition (EMT) is a crucial step for cancer metastasis, LCN2 and DpdtC had opposite effects on EMT markers, the binding of DpdtC to LCN2 significantly weakened the regulation of it (or its iron chelate) on EMT markers. To insight into the interaction between LCN2 and DpdtC–iron, fluorescence titration and molecular docking were performed to obtain the association constant (~ 104 M−1) and thermodynamic parameters (ΔG = − 26.10 kJ/mol). Importantly this study provided evidence that siderophores-loading state of LCN2 may influence its function, which be helpful for understanding the contradictory role of LCN2 in the metastasis of cancer.
Graphic abstract









Similar content being viewed by others
References
Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP (2005) Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J 391:441–448
Bratt T, Ohlson S, Borregaard N (1999) Interactions between neutrophil gelatinase-associated lipocalin and natural lipophilic ligands. Biochim Biophy Acta 1472:262–269
Miharada K, Hiroyama T, Sudo K, Danjo I, Nagasawa T, Nakamura Y (2008) Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol 215(2):526–537
Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K (2008) Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol 181(12):8521–8527
Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS, McCubrey JA, Libra M (2014) Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget 5:1576–1594. https://doi.org/10.18632/oncotarget.1738
Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, Coppolino G, Buemi M (2010) Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett 288:10–16. https://doi.org/10.1016/j.canlet.2009.05.027
Chakraborty S, Kaur S, Guha S, Batra SK (2012) The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 1826:129–169. https://doi.org/10.1016/j.bbcan.2012.03.008
Gomez-Chou SB, Swidnicka-Siergiejko AK, Badi N, Chavez-Tomar M, Lesinski GB, Bekaii-Saab T, Farren MR, Mace TA, Schmidt C, Liu Y, Deng D, Hwang RF, Zhou L, Moore T, Chatterjee D, Wang H, Leng X, Arlinghaus RB, Logsdon CD, Cruz-Monserrate Z (2017) Lipocalin-2 promotes pancreatic ductal adenocarcinoma by regulating inflammation in the tumor microenvironment. Cancer Res 77(10):2647–2660
Chiang KC, Yeh TS, Wu RC, Pang JS, Cheng CT, Wang SY, Juang HH, Yeh CN (2016) Lipocalin 2 (LCN2) is a promising target for cholangiocarcinoma treatment and bile LCN2 level is a potential cholangiocarcinoma diagnostic marker. Sci Rep 6:36138
Ding G, Fang J, Tong S, Qu L, Jiang H, Ding Q, Liu J (2015) Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 75:957–968
Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA (2009) Lipocalin 2 promotes breast cancer progression. Proc Nat Acad Sci USA 106:3913–3918
Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW, Quinn MA, Rice GE (2007) Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer 120:2426–2434. https://doi.org/10.1002/ijc.22352
Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, Ramachandran V, Sung B, Chakraborty A, Bresalier RS, Logsdon C, Aggarwal BB, Krishnan S, Guha S (2008) Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res 68:6100–6108. https://doi.org/10.1158/0008-5472.CAN-08-0540
Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y, Kim JS (2006) Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int J Cancer 118:2490–2497. https://doi.org/10.1002/ijc.21657
Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, Feig B, Zhang W, Pusztai L, Symmans WF, Wu Y, Arlinghaus RB (2009) Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res 69:8579–8584
Lin CW, Yang WE, Lee WJ, Hua KT, Hsieh FK, Hsiao M, Chen CC, Chow JM, Chen MK, Yang SF, Chien MH (2016) Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase IX inhibition and is associated with favourable prognosis. Carcinogenesis 37:712–722
Devireddy LR, Hart DO, Goetz DH, Green MR (2010) A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 141:1006–1017
Arredondo M, Nunez MT (2005) Iron and copper metabolism. Mol Aspects Med 26:313–327
Thévenod F (2018) Iron and its role in cancer defense: a double-edged sword. Met Ions Life Sci 18:437–467
Toyokuni S (2009) Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci 100:9–16
Jung M, Weigert A, Tausendschon M, Mora J, Oren B, Sola A, Hotter G, Muta T, Brune B (2012) Interleukin-10-induced neutrophil gelatinaseassociated lipocalin production in macrophages with consequences for tumor growth. Mol Cell Biol 32:3938–3948
Ören B, Urosevic J, Mertens C, Mora J, Guiu M, Gomis RR, Weigert A, Schmid T, Grein S, Brüne B, Jung M (2016) Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J Pathol 239:274–285
Prill S, Rebstock J, Tennemann A, Körfer J, Sönnichsen R, Thieme R, Gockel I, Lyros O, Monecke A, Wittekind C, Weimann A, Grosser K, Wiechmann V, Kubick C, Bechmann I, Lordick F, Kallendrusch S (2019) Tumor-associated macrophages and individual chemo-susceptibility are influenced by iron chelation in human slice cultures of gastric cancer. Oncotarget 10(46):4731–4742
Jung M, Mertens C, Bauer R, Rehwald C, Brüne B (2017) Lipocalin-2 and iron trafficking in the tumor microenvironment. Pharmacol Res 120:146–156
Mertens C, Mora J, Ören B, Grein S, Winslow S, Scholich K, Weigert A, Malmström P, Forsare C, Fernö M, Schmid T, Brüne B, Jung M (2017) Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology 7(3):e1408751
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK (2002) The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore mediated iron acquisition. Mol Cell 10:1033–1043
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921
Devireddy LR, Gazin C, Zhu X, Green MR (2005) A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123:1293–1305
Richardson DR (2005) 24p3 and its receptor: Dawn of a new iron age? Cell 123:1175–1177
Huang TF, Sun YJ, Li YL, Wang TT, Fu Y, Li CP, Li CZ (2018) Growth inhibition of a novel iron chelator, dpdtC, against hepatoma carcinoma cell lines partly attributed to ferritinophagy-mediated lysosomal ROS generation. Oxid Med Cell Longev 2018:4928703
Li C, Liu Y, Fu Y, Huang T, Kang L, Li C (2017) The antiproliferative activity of di-2-pyridylketone dithiocarbamate is partly attributed to catalase inhibition: detailing the interaction by spectroscopic methods. Mol Biosyst 13(9):1817–1826
Li YL, Huang TF, Fu Y, Wang TT, Zhao TS, Guo S, Yang Y, Li CZ (2019) Antitumor activity of a novel dual functional podophyllotoxin derivative involved PI3K/AKT/mTOR pathway. PLoS ONE 14(9):e0215886
Li CP, Huang TF, Fu Y, Liu YX, Zhou SF, Qi ZY, Li CZ (2016) Interaction of Di-2-pyridylketone 2-pyridinecarboxylic acid hydrazone and its copper complex with BSA: Effect on antitumor activity as revealed by spectroscopic studies. Molecules 21:563
Trott O, Olson AJ (2010) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
DeLano WL (2002) The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002. http://www.pymol.org. Accessed 5 July 2012
Laskowski RA, Swindells MB (2011) LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786
Wang TT, Fu Y, Huang TF, Liu YX, Wu MH, Yuan YB, Li SS, Li CZ (2016) Copper Ion attenuated the antiproliferative activity of di-2-pyridylhydrazone dithiocarbamate derivative; however, there was a lack of correlation between ROS generation and antiproliferative activity. Molecules 21:1088
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410
Conlon GA, Murray GI (2019) Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol 247(5):629–640. https://doi.org/10.1002/path.5225
Lu YN, Dong BJ, Xu F, Xu YZ, Pan JH, Song JJ, Zhang J, Huang YR, Xue W (2019) CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun Signal 17:118
Ding G, Fang J, Tong S, Qu L, Jiang H, Ding Q, Liu J (2015) Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 75(9):957–968
Xu ZJ, Liu YX, Zhou SF, Fu Y, Li CZ (2016) Analysis of the interaction of Dp44mT with human serum albumin and calf thymus DNA using molecular docking and spectroscopic techniques. Int J Mol Sci 17:1042
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. Probe for structural fluctuations inmacromolecules. Biochemistry 12:4161–4170
Bijari N, Shokoohini Y, Ashrafi-Kooshk MR, Ranjbara S, Parvaneh S, Moieni-Arya M, Khodarahmi R (2013) Spectroscopic study of interaction between osthole and human serum albumin: Identification of possible binding site of the compound. J Lumin 143:328–336
Du ZP, Wu BL, Xie YM, Zhang YL, Liao LD, Zhou F, Xie JJ, Zeng FM, Xu XE, Fang WK, Li EM, Xu LY (2015) Lipocalin 2 promotes the migration and invasion of esophageal squamous cell carcinoma cells through a novel positive feedback loop. Biochim Biophys Acta 1853:2240–3225
Chi Y, Remsik J, Kiseliovas V, Derderian C, Sener U, Alghader M, Saadeh F, Nikishina K, Bale T, Iacobuzio-Donahue C, Thomas T, Pe’er D, Mazutis L, Boire A (2020) Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science 369(6501):276–282
Betten R, Scharner B, Probst S, Edemir B, Wolff NA, Langelueddecke C, Lee WK, Thévenod F (2018) Tonicity inversely modulates lipocalin-2 (Lcn2/24p3/NGAL) receptor (SLC22A17) and Lcn2 expression via Wnt/β-catenin signaling in renal inner medullary collecting duct cells: implications for cell fate and bacterial infection. Cell Commun Signal 16(1):74
Wang YP, Yu GR, Lee MJ, Lee SY, Chu IS, Leem SH, Kim DG (2013) Lipocalin-2 negatively modulates the epithelial-to-mesenchymal transition in hepatocellular carcinoma through the epidermal growth factor (TGF-beta1)/Lcn2/Twist1 pathway. Hepatology 58:1349–1361
Kim SL, Lee ST, Min IS, Park YR, Lee JH, Kim DG, Kim SW (2017) Lipocalin 2 negatively regulates cell proliferation and epithelial to mesenchymal transition through changing metabolic gene expression in colorectal cancer. Cancer Sci 108(11):2176–2186
Feng M, Feng J, Chen W, Wang W, Wu X, Zhang J, Xu F, Lai M (2016) Lipocalin2 suppresses metastasis of colorectal cancer by attenuating NF-κB-dependent activation of snail and epithelial mesenchymal transition. Mol Cancer 15(1):77. https://doi.org/10.1186/s12943-016-0564-9
Acknowledgments
This study was supported through grants awarded by the National Natural Science Foundation of China (No. 21571153)and Xinxiang Medical University (provincial) college students' innovation and entrepreneurship project (202010472036).
Author information
Authors and Affiliations
Contributions
CL and YL performed the main experiments; they contributed equally to this work. Changzheng Li conceived and designed the experiments. LL, XH, and HW performed part experiments and data analysis. Tengfei Huang prepared the iron chelator. CL prepared and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing financial interests or any other potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Li, Y., Lou, L. et al. The interaction between lipocalin 2 and dipyridine ketone hydrazone dithiocarbamte may influence respective function in proliferation and metastasis-related gene expressions in HepG2 cell. J Biol Inorg Chem 26, 123–133 (2021). https://doi.org/10.1007/s00775-020-01842-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01842-8