Ultraprocessed food (UPF) consumption has increased significantly in recent decades, reaching more than half of the energy intakes by populations in developed countries(Reference Baraldi, Martinez Steele and Canella1,Reference Rauber, Louzada and Steele2) . In Brazil, the average annual increase of UPF energy contribution has been estimated to be 2·1 % and may reach values between 5 and 10 % in some low-income countries in the coming years(Reference Monteiro, Moubarac and Cannon3).
Evidence points to the UPF’ poor nutritional quality as they tend to be rich in sugar, saturated and trans-fat, Na, with high energetic density and, concomitantly, low in protein, fibres and micronutrients(Reference Rauber, Louzada and Steele2,Reference Monteiro, Levy and Claro4–Reference Marrón-Ponce, Tolentino-Mayo and Hernández-F11) . In Brazil, estimates indicate greater UPF consumption during childhood suggesting that children are eating a poor-quality diet in a period considered crucial for establishing healthy eating habits(Reference Savage, Fisher and Birch12,Reference Vitolo, Rauber and Campagnolo13) . Such a situation may be partly responsible for the rise in the prevalence of overweight/obesity and systemic arterial hypertension observed among Brazilian children and adolescents(Reference Lelis, Teixeira and Silva14).
There is consensus that a healthy diet in early life, including exclusive breast-feeding (BF) up to 6 months and complemented BF up to 2 years or more, is key to establishing healthy eating habits(Reference Savage, Fisher and Birch12,Reference Vitolo, Rauber and Campagnolo13) . On the other hand, adolescent motherhood can negatively affect BF initiation and duration, as well as prompt the consumption of unhealthy foods in infants’ early(Reference Lauzon-Guillain, Jones and Oliveira15).
We are not aware of studies evaluating the effect of educational dietary interventions early in life on UPF consumption in the medium or long term. In this context, we aimed to evaluate the effect of an educational intervention to promote BF and healthy complementary feeding among adolescent mothers in the first months of infants’ lives, on the consumption of UPF at the age of 4–7 years.
Methods
Design and population
This randomised clinical trial (RCT) enrolled 323 adolescent mothers, their infants and the maternal grandmothers when cohabiting. Mothers were recruited from the maternity ward of a teaching hospital, certified by the Baby-Friendly Hospital Initiative in South Brazil. On a daily basis, including weekends, mothers who met the inclusion criteria were identified: those living in Porto Alegre city; 19 years old or younger; single birth and who initiated BF post-childbirth. The exclusion criteria were: mothers and newborns physically separated; maternal or neonatal complications; low birth weight (<2500 g) and mothers who cohabited with the paternal child’s grandparents; as well as situations that impeded BF, as the AIDS (HIV) and congenital malformations.
Sample calculation
Since the original clinical trial was planned to evaluate another question (exclusive BF and overall BF duration rates in the infants’ first year of life), we calculated the effect size that can be detected with the sample available at the follow-up assessment (n 194), according to the new question. Considering the estimate that 37·7 % of the children in the control group have a high consumption of UPF(Reference Cediel, Reyes and Da Costa Louzada8), a sample size of 102 children in the control group and ninety-two children in the intervention group can detect relative risk in favour of the intervention of up to 0·519, that is, a maximum risk reduction of 48·1 %, with 5 % significance and 80 % power. Such calculation was performed in software R using the epiR package.
Randomisation and study steps
The eligible adolescents’ mothers were allocated to the intervention or control groups by a random draw, using two spheres of the same texture and dimensions with the words ‘yes’ and ‘no’, which were removed from a dark-coloured casing by the same researcher who made the initial enrolment. Randomisation was performed in blocks of two, so if one mother was drawn into the intervention group, the next eligible mother was included in the control group.
The study was conducted in two phases: (1) the first was between 2006 and 2008 with the intervention and follow-up to 12 months and (2) follow-up visit at the age of 4–7 years, between 2012 and 2013, assessing child’s health. The flow chart of the phases of the RCT is shown in Fig. 1.

Fig. 1. Flow chart of the randomised clinical trial phases from sample selection to 4–7 years.
Intervention
Intervention sessions occurred at the maternity ward, within 72 h after delivery, and at the mothers’ homes, at 7, 15, 30, 60 and 120 d of infants’ life. Each session lasted approximately 30–40 min and was conducted by members of a team composed of two nurses, one nutritionist and one paediatrician, three of them certified by the International Board Lactation Consultant Examiners, conducted the intervention using communication skills, such as motivational interviewing, recommended by the WHO.
During the first session, the consultant and the mother and/or grandmother, the latter when cohabiting, talked about several aspects related to BF practices, for example, its importance; frequency and recommended duration; factors that interfere in milk supply; BF techniques; consequences of dummy use and bottle-feeding; infant crying and communication and specific doubts expressed by the mothers and/or grandmothers. Mothers were also encouraged to breastfeed, when appropriate, in order to provide the interviewer with an opportunity to observe the BF and provide guidance on proper positioning and handling. In addition, support material was delivered, which included an illustrated booklet and a serial album designed for the study intervention. The materials were developed after a pilot study conducted previously with adolescent mothers focus groups, adapting the language and content for this specific population. All mothers, regardless of group allocation, received standard maternity care.
Subsequent visits, at mothers’ home, were used to reinforce messages originally conveyed during initial counselling with the help of the support material delivered in the first session, and mainly to discuss the challenges related to infant feeding.
The 120-d intervention session emphasised the introduction of healthy complementary feeding after 6 months of life, as recommended by the Brazilian guidelines(16). In this session, we discussed the type, variety, consistency and hygiene of the foods to be offered, in addition to the delivery of a second booklet with more information on the topics covered and recipes to exemplify food groups and food preparation.
Follow-up visit at 4–7 years
One last evaluation took place from September 2012 to July 2013, when children were aged 4–7 years, at the Hospital de Clínicas de Porto Alegre Research Center (or at home when mothers and children failed to attend the centre). After obtaining the mothers’ informed consent, they were interviewed about the current demographic and socio-economic situation, in addition to performing anthropometry. For the anthropometric evaluation, two measures of weight and height of each child were taken using standard techniques(17). We used the WHO reference and cut-off points for BMI for age and height for age classifications(Reference Onis, Onyango and Borghi18). At this time, the first 24-h food recall was applied. Two subsequent 24-h food recall were collected by telephone, on two different days from the same week, including at least 1 d on the weekend. Data collection and anthropometric assessment were always carried out by three graduate students (two master’s students and one doctoral student); researchers were blinded to group allocation and not participating in the data collection of the first phase of the study.
Dietary intakes
Dietary intakes were obtained through up to three 24-h food recall and analysed in ADSNutri 2006® software. The nutrients were calculated based on the Brazilian Food Composition Table(19), as well on the United States Department of Agriculture Table(20) and, in some cases, on the information contained in the product label.
The home-made measures reported were transformed into food grams based on national reference tables(Reference Pinheiro, Lacerda and Benzecry21,Reference Bombem, Canella and Bandoni22) , and the food preparations that were not available in ADSNutri® were entered manually, using standardised recipes(Reference Pinheiro, Lacerda and Benzecry21,Reference Bombem, Canella and Bandoni22) . A standardisation manual was prepared when, eventually, there was no brand, size or description of the recipe in the 24-h food recall.
Food items were classified into mutually exclusive NOVA groups(Reference Monteiro, Cannon and Moubarac23) and subgroups by two independent researchers and reviewed by a panel of experts from the Center for Epidemiological Research in Nutrition (NUPENS). Discrepancies were discussed until consensus was reached. We decided not to break down culinary recipes into their underlying ingredients due to lack of detailed information on ingredients brands and contents.
We calculated the contributions of each NOVA food group to the total energy consumption (% E), and the UPF group was distributed in tertiles, the highest tertile being considered high consumption and the second and first tertiles as low consumption. The subgroups were presented in g of food.
Statistical analysis
We compared the characteristics of the control and intervention groups. The Student’s t or Mann–Whitney U tests were used when appropriate for comparison of quantitative variables, and the Pearson χ 2 or Fisher’s exact tests were used for comparison of categorical variables.
Dietary contribution of UPF (% of total energy intake) was adjusted for intra-individual variability by the SPADE method(Reference Dekkers, Verkaik-Kloosterman and van Rossum24), considering the child’s age to represent usual consumption. Such values were described by means of median, interquartile range, minimum and maximum. For comparison, the average percentage UPF for each child was calculated.
We used Poisson regression models with robust variance to estimate the effects of the intervention and BF duration on the prevalence of high UPF consumption (highest tertile), compared with low consumption (other two tertiles). The intervention and control groups were not stratified by maternal grandmothers’ cohabitation, considering it did not influence previously published results of the RCT(Reference Nunes, Vigo and de Oliveira25,Reference Soldateli, Vigo and Giugliani26) . Model 1 (crude model) is univariable. Then, a series of cumulative models were performed by the sequential addition of covariates. In model 2, BF duration was added. In the third model, we included a propensity score, which was estimated through logistic regression, to model the probability of an individual being allocated to the intervention group and considering the following predictors: age, maternal education and colour skin, birth weight and sex of the child (all variables from the study baseline). The propensity score was intended to be an additional resource to control for potential confounding caused by an imbalance between groups given that randomisation was carried out in 2006, and there were losses through 2013. In model 4, income was included. Finally, model 5 was adjusted for total energy content.
The same adjusted models were used to estimate the effect of BF duration on high UPF consumption (highest tertile). In all models, the relative risk and its 95 % CI were obtained, using the R software version 3.6.0. The level of significance was set at 5 % (P ≤ 0·05).
Ethics
This study was conducted in accordance with the Guidelines for Health Research (Ordinance 01/88 National Health Congress, supplemented by Resolution 466/2012). The research was approved by the Scientific Committee and Research Ethics Committee in Health of the Clinic Hospital Porto Alegre and by Plataforma Brazil (120249). The clinical trial was registered in ClinicalTrials.gov, under number NCT00910377.
Results
One hundred ninety-four children completed the study (Fig. 1). Table 1 shows maternal and children’s baseline and at age 4–7 years characteristics and according to intervention/control groups. Most of the mothers declared themselves white, average schooling of 8·9 (sd 2·2) years and average family income per capita below the minimum wage. Regarding children’s characteristics, 51 % were female in the follow-up, the average age was 6·1 (sd 0·5) years and 34·4 % were overweight.
Table 1. Characteristics of the sample
(Numbers and percentages; mean values and standard deviations)
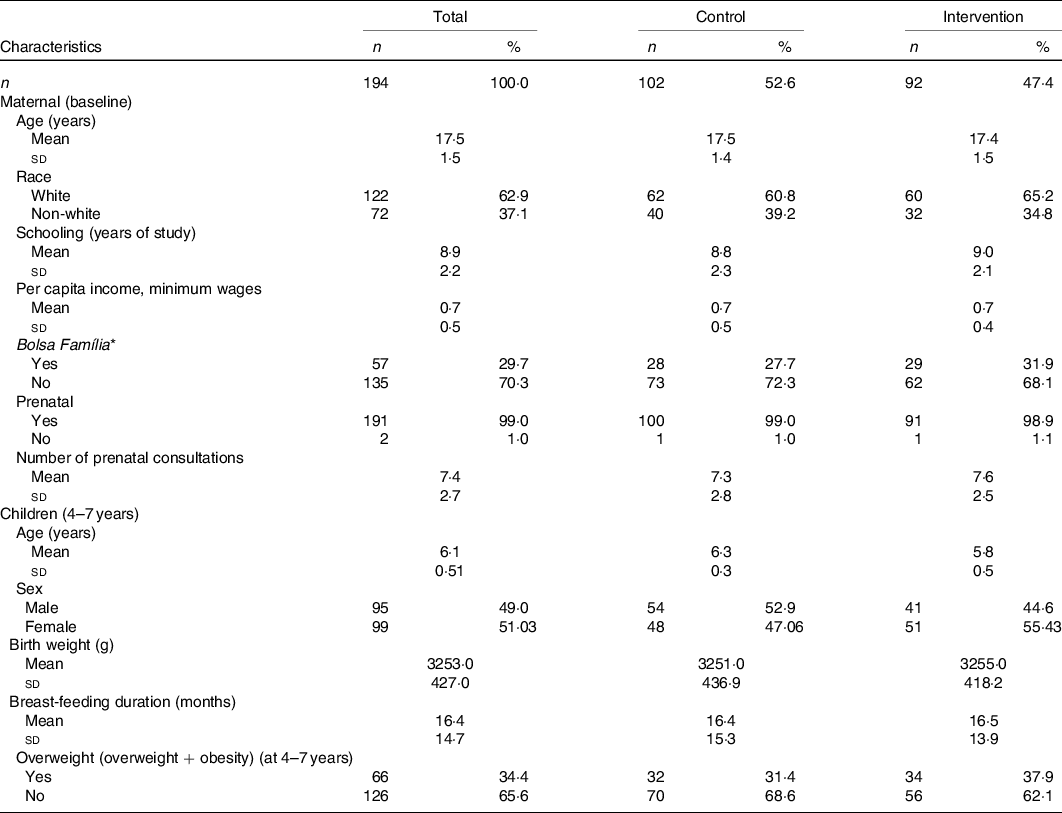
* Income aid provided by the Brazilian government.
Table 2 shows UPF, energy and macronutrient intakes according to intervention/control groups. The median of UPF corrected by the average between the 24-h recall for each child was 40·1 % (interquartile range 30·1, 51·3) and by the SPADE method was 42·7 % (interquartile range 38·0, 42·7). The intervention group presented slightly lower medians of UPF (37·5 and 38·0 %) compared with the control group (42·3 and 42·7 %) in both methods for intra-individual correction. In the last tertile of UPF consumption (defined as high UPF consumption), the energy contribution of UPF varied between 48·6 and 100 %. The average energetic consumption was similar between groups, as well as the percentage of macronutrients. Table 2 also describes the most commonly consumed NOVA unprocessed or minimally processed (G1) and UPF (G4). The consumption of fruits, vegetables and meat/eggs was slightly higher in the intervention group compared with the control group. High consumption of sugar drinks (soft drinks and artificial juices) was observed in both groups. Except for biscuits, processed meats and products like margarine, jellies and processed cheeses, the other UPF subgroups showed higher consumption in the control group.
Table 2. Food consumption according to groups* 1 and 4 of the NOVA classification and contribution of ultraprocessed foods (UPF) to total energy intake, macronutrients and micronutrients (n 194)
(Mean values and standard deviations; medians and interquartile ranges (IQR); minimum (min) and maximum (max) values)
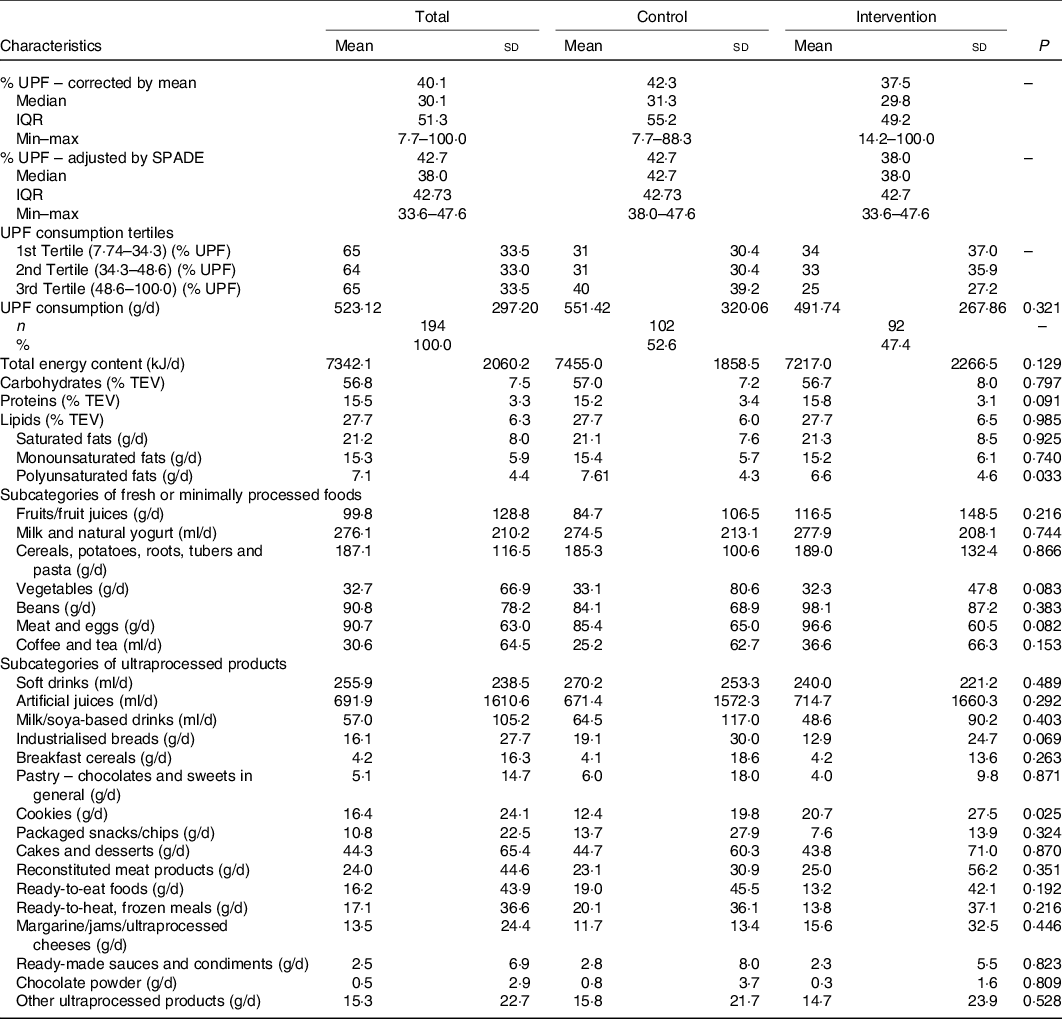
TEV, total energy value.
* Group 1: subcategories of fresh or minimally processed foods; group 4: subcategories of ultraprocessed foods.
Table 3 shows the Poisson regression models with robust variance for the effect of the intervention on high UPF consumption (defined as the highest tertile of UPF consumption). The final model adjusted for BF duration, propensity score, income and total energy content shows that the intervention reduced the risk of high UPF consumption by 35 % (relative risk 0·65, 95 % CI 0·43, 0·98) (P = 0·04). We did not find an association between BF duration and high UPF consumption at age of 4–7 years.
Table 3. Poisson regression models with robust variance assessing the effect of the intervention and breast-feeding (BF) duration on high consumption of ultraprocessed products*
(Relative risks (RR) and 95 % confidence intervals)

* The last tertile is the outcome, it was considered a high consumption of ultraprocessed products.
† Variables included in the propensity score (from the study baseline): maternal age, education and skin colour; birth weight and infant sex.
Discussion
Our results demonstrated that an educational intervention to promote BF and healthy complementary feeding target to adolescent mothers in the first months of infants’ lives reduced by 35 % the risk of high consumption of UPF at child’s age of 4–7 years. However, we did not find an effect of BF duration on high UPF consumption. The UPF consumption, especially sugary drinks (soft drinks and artificial juices), was high in this age group. The frequency of consumption of dairy beverages, packaged snacks, ready-to-eat and ready-to-heat foods was also high, being higher in the control group. In contrast, fruits and vegetables were more consumed by the intervention group.
The positive effect of the intervention performed early in life on food consumption at the age of 4–7 years found in this study is consistent with the results of previous studies. In Brazil, another RCT evaluated the impact of an educational dietary intervention carried out from puerperium until the end of the first year of life and reported repercussions on better eating practices at preschool age, though loosing effect at the age of 8 years(Reference Vitolo, Rauber and Campagnolo13,Reference Rauber, Hoffman and Vitolo27) . A meta-analysis that included twenty-one clinical trials with children aged 5–12 years showed that interventions in this age group to promote fruits and vegetable consumption obtained modest improvements in dietary practices, suggesting that interventions carried out after the first year of life might not be as effective, reinforcing the importance of earlier interventions such as the present study(Reference Evans, Christian and Cleghorn28).
There are still few studies evaluating the association between BF duration and the consumption of UPF in preschoolers. We acknowledge only one study assessing the association between exclusive BF duration and UPF intakes in children aged 4–7 years, which demonstrated a 0·7 % decrease in energy intakes from UPF for each month of exclusive BF duration(Reference Fonseca, Ribeiro and Andreoli29). In the present study, this association was not statistically significant. However, research has shown a positive relationship between BF duration, the introduction of healthy complementary foods and infant feeding quality in the first years of life and in the preschool phase, especially concerning the consumption of fruits and vegetables(Reference Armstrong, Abraham and Squair30,Reference Rauber, Campagnolo and Hoffman31) .
The high percentage energy intakes from UPF observed in our study are consistent with values observed in other Brazilian studies such as Fonseca et al. (Reference Fonseca, Ribeiro and Andreoli29) and Costa et al. (Reference Costa, Del-Ponte and Assunção32), which reported 38 and 41·8 % of total energy percentage, respectively. Although the consumption of UPF is still lower than that of developed countries, which may reach 65 % of the energy intake(Reference Martines, Machado and Neri33), in this study, we can see a higher UPF consumption than in countries such as Chile, with 28·6 %(Reference Cediel, Reyes and Da Costa Louzada8), and Colombia, with 15·9 %(Reference Parra, da Costa-Louzada and Moubarac10). Evidence indicates that an inadequate diet in childhood is determined by maternal age(Reference Navia, Ortega and Rodríguez-Rodríguez34), maternal education(Reference Dubois, Farmer and Girard35) and family income(Reference Kranz, Findeis and Shrestha36). Therefore, our sample profile can explain the high UPF consumption percentages in children since they belong to low-income families, and their adolescent mothers presented few years of schooling.
Similar to our findings, other studies also observed that one of the highest UPF energy contributors in childhood is sweetened drinks such as soft drinks(Reference Scharf and DeBoer37). In a sensitivity analysis, removing the ultraprocessed sweetened beverages from the UPF group, we no longer found a significant protective effect of the intervention (relative risk 0·69, 95 % CI 0·47, 1·04; P = 0·082). This is an important finding since other studies suggest associations between sweetened drinks and adverse health outcomes, such as for overweight, obesity, hypertension, type 2 diabetes and CVD in children and adults(Reference Malik, Pan and Willett38,Reference Vartanian, Schwartz and Brownell39) .
The high percentage of energy intake from UPF may be one reason why another study carried out with the same sample failed to observe a protective effect of BF against overweight(Reference Schwartz, Vigo and DeOliveira40). Considering the multi-causality of overweight, the high consumption of UPF in this population may have hidden the positive impact of BF on nutritional status(Reference Schwartz, Vigo and DeOliveira40). An RCT found that the consumption of a diet rich in UPF promotes higher total energy intakes, about 2092 kJ (500 kcal) more per d, further confirming the relationship between UPF and obesity(Reference Hall, Ayuketah and Brychta41).
Some limitations in the present study must be considered, such as losses on follow-up, which accounted for 39·2 % at 4–7 years. The losses are due to the typical mobility of the young population living in peripheral areas of cities in developing countries(Reference Rothman42). However, the study design and the inclusion of the propensity score in the analysis minimised this limitation. Moreover, variables known to be associated with children’s eating habits, such as schooling and maternal age and family income, remained similar between groups at baseline and in follow-up at 4–7 years.
Though 24-h recalls are considered the least biased method to describe dietary intake at the population level(Reference Prentice and Huang43), social desirability bias may lead to an underestimation of UPF consumption (especially absolute values but potentially also relative ones). This most likely non-differential bias may underestimate the association between intervention/control and high UPF consumption.
Although this study collected some information indicative of the type of processing to which foods were submitted before consumption or culinary preparation (i.e. place of meals, product brands), this information was not available for all food items, which could lead to modest over- or underestimation of UPF consumption.
The study’s strengths are the reduced possibility of contamination between groups in the maternity ward since an average of one or two mothers was selected per d, with few chances of exchanging information, in addition to the fact of the intervention being performed in a private environment. Moreover, the interviewers at 4–7 years were blinded to the groups.
Finally, this study demonstrated that an intervention to promote BF and complementary healthy feeding introduction, target to adolescent mothers in the first months of infants’ lives, was effective in reducing the risk of high consumption of UPF at 4–7 years; however, it was not possible to demonstrate the association with the BF duration. The high consumption of UPF in both groups shows the increased participation of these foods in children’s diets. We suggest that further studies should include more follow-up visits to set up interventions in the middle ages, also measuring the mothers’ nutritional intakes and nutritional status of both in all visits.
Acknowledgements
The authors are grateful to the participants of the study.
This study received financial support from the Fund for Research and Event Promotion – Hospital de Clínicas de Porto Alegre. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. E. M. S. received funding from FAPESP: Fundação de Amparo à Pesquisa do Estado de São Paulo (Processo FAPESP no. 2018/17972-9).
B. L., B. S. and M. D. contributed to concept development and interpreted the data. V. B. L. and L. G. P. conducted the analysis. B. L. and L. G. P. were responsible for execution of the study and processing the data. B. L. drafted the manuscript. B. S., V. B. L., E. R. G., C. A. M., E. M. S. and M. D. critically reviewed the manuscript. All authors have responsibility for the final content.
The authors declare that there are no conflicts of interest.









