Abstract
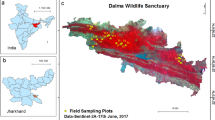
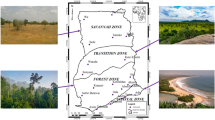
In view of increasing anthropogenic influences and global changes, quantification of carbon assimilation through photosynthesis has gained tremendous significance. Precise estimation of Gross Primary Productivity (GPP) is essential for several ecosystem models and is typically done using coarser scale satellite data. The mangrove ecosystem, which offers significant protection to the coastal environment, is one of the critical habitats from a global change point of view. Light use efficiency (LUE) was measured using diurnal in situ photosynthetic rate observations for 13 dominant mangrove species for 3 seasons at each of the three mangrove dominant test-sites situated along the east and west coast of India. Variations in photosynthetic rates among these species were studied for 3 seasons that indicated varying responses of mangrove ecosystem at each site. Among all species, Rhizophora mucronata and Sonneratia apetala indicated higher values at two of the test-sites. IRS Resourcesat-2 LISS-IV datasets were used for the estimation of GPP. Mean GPP for all the sites varied from 1.2 to 7.7 g C m−2 day−1 with maximum value of 14.4 g C m−2 day−1. Mean values of GPP varied across the sites, based on its maximum LUE values and available photosynthetically active radiation (PAR). The results provide GPP values at much better spatial resolution for a threatened habitat like mangroves that typically survive in a narrow habitat along the coasts.















Similar content being viewed by others
References
Alongi, D. M. (1994). The role of bacteria in nutrient recycling in tropical mangrove and other coastal benthic ecosystems. Hydrobiologia, 285, 19–32.
Alongi, D. M. (2002). Present state and future of the world’s mangrove forests. Environmental Conservation, 29, 331–349.
Alongi, D. M. (2009). The energetics of mangrove ecosystem. Dordrecht: Springer.
Ball, M. C. (1981). Physiology of photosynthesis in two mangrove species: responses to salinity and other environmental factors. Ph.D. Thesis. Australian National University, Canberra.
Ball, M. C. (1986). Photosynthesis in mangroves. Wetlands Australia, 6(1), 12.
Ball, M. C. (1988). Ecophysiology of mangroves. Trees, 2, 129–142.
Ball, M. C., & Anderson, J. M. (1986). Sensitivity of photosystem II to NaCl in relation to salinity tolerance. Comparative studies with thylakoids of the salt-tolerant mangrove, Avicennia marina, and the salt-sensitive pea, Pisum sativum. Australian Journal of Plant Physiology, 13, 689–698.
Ball, M. C., & Critchley, C. (1982). Photosynthetic responses to irradiance by the grey mangrove, Avicennia marina, grown under different light regimes. Plant Physiology, 74, 7–11.
Ball, M. C., & Farquhar, G. D. (1984). Photosynthetic and stomatal response of two mangrove species Aegiceras corniculatum and Avicennia marina to long term salinity and humidity conditions. Plant Physiology, 74, 1–6.
Barr, J. G., Engel, V., Fuentes, J. D., Fuller, D. O., & Kwon, H. (2013). Modelling light use efficiency in a subtropical mangrove forest equipped with CO2 eddy covariance. Biogeosciences, 10, 2145–2158.
Chapin III, F. S., & Eviner, V. T. (2007). Biogeochemistry of terrestrial net primary production. Treatise on Geochemistry, 8, 1–35.
Cheeseman, J. M., Clough, B. F., & Carter, D. R. (1991). The analysis of photosynthetic performance in leaves under field conditions: a case study using Bruguiera mangroves. Photosynthesis Research, 29, 11–22.
Cheeseman, J. M., Herendeen, L. B., Cheeseman, A. T., & Clough, B. F. (1997). Photosynthesis and photoprotection in mangroves under field conditions. Plant, Cell & Environment, 20, 579–588.
Choudhury, B. J. (1987). Relationship between vegetation indices, radiation absorption and net photosynthesis evaluated by a sensitivity analysis. Remote Sensing of Environment, 22, 209–233.
Clough, B. F., Andrews, T. J., & Cowan, I. R. (1982). Physiological process in mangroves. In B. F. Clough (Ed.), Mangrove Ecosystems in Australia: Structure, Function and Management (pp. 193–210). Canberra: Australian National University Press.
Clough, B. F. (1984). Growth and salt balance of the mangroves Avicennia marina (Forsk.) Vierh. and Rhizophora stylosa Griff. in relation to salinity. Australian Journal of Plant Physiology, 11, 419–430.
Donato, D. C., Kauffman, J. B., & Murdiyarso, D. (2011). Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience, 4, 293–297.
Gitelson, A. A., & Gamon, J. A. (2015). The need for a common basis for defininfg light use efficiency: Implications for productivity estimation. Remote Sensing of Environment, 42, 187–216.
Gnanamoorthy, P., Selvam, V., Chakraborty, S. & Debburman, P. (2017). Eddy covariance measurement of carbon dioxide (CO2) exchange in Pichavaram mangrove ecosystem, southeast coast of India. Proceedings of the 22nd international forestry and environment symposium 2017 of the Department of Forestry and Environmental Science, University of Sri Jayewardenepura, Sri Lanka.
Goetz, S. J., & Prince, S. D. (1999). Modeling terrestrial carbon exchange and storage: Evidence and implications of functional convergence in light use efficiency. Advances in Ecological Research, 28, 57–92.
Hamilton, J. G., Delucia, E. H., George, K., Naidu, S. L., Finzi, A. C., & Schlesinger, W. H. (2002). Forest carbon balance under elevated CO2. Oecologia, 131(2), 250–260.
Heinsch, F. A., Zhao, M., Running, S. W., Kimball, J., Nemani, R., Davis, K., et al. (2006). Evaluation of remote sensing based terrestrial productivity from MODIS using tower eddy flux network observations. IEEE Transactions on Geoscience and Remote Sensing, 44, 1908–1925.
Janzen, H. H. (2004). Carbon cycling in earth systems - a soil science perspective. Agriculture, Ecosystems and Environment, 104(3), 399–417.
Kathiresan, K., & Kannan, L. (1985). Photosynthetic productivity in species of Rhizophora species. The Mangroves (pp. 262–265). Kolhapur, India: Proc. Natl. Symp. Biol. Util. Cons. Mangroves at Shivaji University.
Kathiresan, K., & Moorthy, P. (1993). Influence of different irradiance on growth and photosynthetic characteristics in seedlings of Rhizophora species. Photosynthetica, 29, 143–146.
Kathiresan, K., & Moorthy, P. (1994). Photosynthetic response of Rhizophora apiculata Blume to long-chain aliphatic alcohol. Aquatic Botany, 47, 191–193.
Krauss, K. W., & Allen, J. A. (2003). Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquatic Botany, 77, 311–324.
Kripa, M. K., Gohel, A., Lele, N., Mankad, A. U., & Murthy, T. V. R. (2019). Modelling the gross primary productivity (GPP) of an Indian mangrove vegetation. Environment and Ecology, 37(1A), 221–228.
Kumar, T., Murthy, T. V. R., Chellamani, P., Krishnan, V., Thangaradjou, T., & Murali, M. (2017). Modeling photosynthetic rates of Indian red mangroves (Rhizophora mucronata Poir.) to climatic factors. Tropical Ecology, 72–79.
Landsberg, J. J. (1986). Physiological ecology of forest production (pp. 165–178). London: Academic press.
Law, B. E., Anthoni, P. M., & Aber, J. (2000). Measurements of gross and net ecosystem productivity and water vapor exchange of a Pinus ponderosa ecosystem, and an evaluation of two generalized models. Global Change Biology, 6, 155–168.
Lele, N., Kripa, M. K., & Murthy, T. V. R. (2017). Quantifying the GPP of an Indian mangrove forest using GEO- LEO satellites. Proc. 38th Asian Conference of Remote Sensing, 23-27 October 2017, New Delhi (India).
Monteith, J. (1972). Solar radiation and productivity in tropical ecosystems. Journal of Applied Ecology, 9, 747–766.
Moore, R. T., Miller, P. C., Ehleringer, J., & Lawrence, W. (1973). Seasonal trends in gas exchange characteristics of three mangrove species. Photosynthetica, 1, 387–394.
Moorthy, P., & Kathiresan, K. (1999). Photosynthetic efficiency of Rhizophoracean mangroves with reference to compartmentalization of photosynthetic pigments. Revista de Biologica Tropical, 47(1-2), 21–25.
Myneni, R. B., & Williams, D. L. (1994). On the relationship between FAPAR and NDVI. Remote Sensing of Environment, 49(3), 200–211.
Nandy, P., & Ghose, M. (2001). Photosynthesis and water use efficiency of some mangroves from Sundarbans India. Journal of Plan Biology, 44(4), 213–219.
National Wetland Atlas. (2011). Report no. SAC/ EPSA/ ABHG/ NWIA/ ATLAS/ 34/ 2011S. Space applications Centre (ISRO), Ahmedabad, India.
Nichol, C. J., Huemmrich, K. F., Black, T. A., Jarvis, P. G., Walthall, C. L., Grace, J., & Hall, F. G. (2000). Remote sensing of photosynthetic-lightuse efficiency of boreal forest. Agricultural and Forest Meteorology, 101, 131–142.
Okimoto, Y., Nose, A., Ikeda, K., Agarie, S., Oshima, K., Tateda, Y., Ishii, T., & Nhan, D. D. (2008). An estimation of CO2 fixation capacity in mangrove forest using to methods of CO2 gas exchange and growth curve analysis. Wetlands Ecology and Management, 16, 155–171.
Ong, J. E. (1993). Mangrove - a carbon source and sink. Chemosphere, 27, 1097–1107.
Peng, D., Zhang, B., Liu, L., Fang, H., Chen, D., Hu, Y., & Liu, L. (2012). Characteristics and drivers of global NDVI-based FPAR from 1982 to 2006. Global Biogeochemical Cycles, 23(3), GB3015.
Polidoro, B. A., Carpenter, K. E., Collins, L., Duke, N. C., Ellison, A. M., Ellison, J. C., et al. (2010). The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS One, 5, 1–10.
Pool, D. J., Lugo, A. E., & Snedaker, S. C. (1975). Litter production in mangrove forests of southern Florida and Puerto Rico. In G. E. Walsh, S. C. Snedaker, & H. J. Teas (Eds.), International Symposium on Biology and Management of Mangroves (pp. 213–237). Gainesville: Institute of Food and Agricultural Sciences, University of Florida.
Potter, C. S., Randerson, F., Field, C. B., Matson, P. A., Vitousek, P. M., Mooney, H. A., et al. (1993). Terrestrial ecosystem production: a process model based on global satellite and surface data. Global Biogeochemical Cycles, 7, 811–841.
Prince, S. D. (1991). A model of regional primary production for use with coarse resolution satellite data. International Journal of Remote Sensing, 12, 1313–1330.
Prince, S. D., & Goward, S. J. (1995). Global primary production: A remote sensing approach. Biogeogr., 22, 815–835.
Quartel, S., Kroon, A., Augustinus, P. G. E. F., Van Santen, P., & Tri, N. H. (2007). Wave attenuation in coastal mangroves in the Red River delta, Vietnam. Journal of Asian Earth Sciences, 29(4), 576–584.
Rodda, S. R., Thumaty, K. C., Jha, C. S., & Dadhwal, V. K. (2016). Seasonal variations of carbon dioxide, water vapor and energy fluxes in tropical Indian mangroves. Forests, 7(2), 35.
Running, S.W., Baldocchi, D.D., Turner, D.P., Gower, S.T., Bakwin, P.S. & Hibbard, K.A. (1999). A global terrestrial monitoring network integrating tower fluxes, flask sampling, ecosystem modeling and EOS satellite data. Remote Sensing of Environment, 70, 108–127
Running, S. W., Thornton, P. E., Nemani, R., & Glassy, J. (2000). Global terrestrial gross and net primary productivity from the earth observing system. In O. E. Sala, R. B. Jackson, & H. A. Mooney (Eds.), Methods in ecosystem science (pp. 44–57). New York: Springer-Verlag.
Running, S. W., Nemani, R. R., Heinsch, F. A., Zhao, M., Reeves, M., & Hashimoto, H. (2004). A continuous satellite-derived measure of global terrestrial primary production. Bioscience, 54, 547–560.
Schaeffer-Novelli, Y. G., Clintron-Molero, R. R. A., & Camargo, T. M. (1990). Variability of mangrove ecosystem along the Brazilian coast. Estuaries, 13, 204–218.
Sellers, P. J. (1985). Canopy reflectance, photosynthesis and transpiration. International Journal of Remote Sensing, 6, 1335–1372.
Sellers, P. J. (1987). Canopy reflectance, photosynthesis and transpiration, II. The role of biophysics in the linearity of their independence. Remote Sensing of Environment, 21, 143–183.
Sellers, P. J., Berry, J. A., Collatz, G. J., Field, C. B., & Hall, F. G. (1992). Canopy reflectance, photosynthesis and transpiration III A reanalysls using enzyme kineticselectron transport models of leaf phvslology. Remote Sensing of Environment, 42, 187–216.
Sellers, P. J., Los, S. O., Tucker, C. J., Justice, C. O., Dazlich, D. A., James Collatz, G., & David, A. (1996). Randall. A revised land surface parametrization (SiB2) for atmospheric GCMs. Part II: the generation of global field of terrestrial biophysical parameters from satellite data. Journal of Climate, 9, 722.
Twilley, R. R., Chen, R., & Hargis, T. (1992). Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water, Air, and Soil Pollution, 64, 265–288.
Twilley, R. R., Pozo, M., Garcia, V. H., Rivera-Monroy, V., Zambrano, R., & Bodero, A. (1997). Litter dynamics in riverine mangrove forests in the Guayas river estuary, Eucador. Oecologia, 111, 109–122.
Weiss, M & Barret, F. (2010). FAPAR (fraction of absorbed photosynthetically active radiation) estimates at various scales. In Proceedings of the 34th International Symposium on Remote Sensing and Environment (ISRSE), Sydney, Australia, 10–15 April 2010. pp 1–4.
Wongpattanakul, P., Ritchie, R. J., Koedsin, W., & Suwanprasit, C. (2015). Photosynthetic rates in mangroves. International Conference on Plant, Marine and Environmental Sciences (PMES-2015) Jan. 1-2, 2015 Kuala Lumpur (Malaysia).
Xiao, X., Hollinger, D., & Aber, J. (2004). Satellite-based modeling of gross primary production in an evergreen needle leaf forest. Remote Sensing of Environment, 89, 519–534.
Xiao, X., Zhang, Q., & Hollinger, D. (2005). Modelling gross primary production of an evergreen needle leaf forest using MODIS and climate data. Ecological Applications, 15, 954–969.
Yamamoto, H. Y. (1979). Biochemistry of violaxanthin cycle. Pure and Applied Chemistry, 51, 639–648.
Zhao, M. S., Heinsch, R., Nemani, R., & Running, S. W. (2005). Improvement of the MODIS terrestrial and gross primary production global data set. Remote Sensing of Environment, 95(2), 164–176.
Acknowledgments
The activity was carried out under PRogrAmme for Climate change Research In Terrestrial envIronment (PRACRITI) in Space Applications Centre (SAC), ISRO (Govt. of India). The authors are grateful to ISRO Headquarters and Director SAC for all the necessary support throughout the programme. The authors are thankful to Deputy Director, EPSA, for the necessary support and encouragement. Support from State Forest departments of Odisha, Tamil Nadu and Goa for necessary permissions and conducting ground experiments are highly obliged. The authors also thank the anonymous reviewers for their keen comments.
Funding
Department of Space, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lele, N., Kripa, M.K., Panda, M. et al. Seasonal variation in photosynthetic rates and satellite-based GPP estimation over mangrove forest. Environ Monit Assess 193, 61 (2021). https://doi.org/10.1007/s10661-021-08846-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08846-0