Abstract
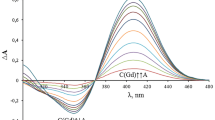
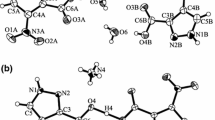
This work continues a series of studies devoted to complex formation of ions of biogenic metals with the flavonoid dihydroquercetin (DHQ). The interaction of Со2+ ions with DHQ in aqueous solutions has been investigated. It has been found that, at different pH of a solution, complex compounds (CC) with different stoichiometry are formed; a variation of the pH value of a solution from 6.0 to 7.0 results in the formation of compounds (1)–(3) with the metal : flavonoid ligand ratio (Met : L) from 1 : 2 at рН 6.0 (1), through 2 : 3 at pH 6.4–6.7 (2), to 1 : 1 at рН 6.8–7.0 (3). By using the thermogravimetric method and the data of the elemental analysis, the most probable composition of the compounds with the determination of the amount of bound water has been proposed: [CoL2(H2O)4] for (1), [Co2L3(ОН)(H2O)4] for (2), and [CoL(ОН)(H2O)2] for (3). Conditions for the optimization of product yield in the complexation reaction of Со2+ ions with DHQ in an aqueous solution have been determined for compound (2): the рН value of solution 6.7; the reaction time 15 min; the temperature of the reaction solution 90°С; the molar ratio of the initial reagents DHQ : Со2+ 1 : 1.5; the initial concentration of DHQ 0.020 mol/L and that of Со2+ 0.030 mol/L; and the use of CoSO4 ⋅ 7H2O as a source of cobalt ions. The yield of the product is 81.8%.


Similar content being viewed by others
REFERENCES
Trofimova, N.N., Stolpovskaya, E.V., Babkin, V.A., Fedorov, S.V., Kalabin, G.A., Goryainov, S.V., Zolotarev, E.E., Safronov, A.Yu., Kashevskii, A.V., and Zhitov, R.G., The structure and electrochemical properties of metal complexes with dihydroquercetin, Russ. J. Bioorg. Chem., 2015, vol. 41, no. 7, pp. 745–752. https://doi.org/10.1134/S1068162015070146
Stolpovskaya, E.V., Trofimova, N.N, and Babkin, V.A., Assessment of the antiradical activity of dihydroquercetin complexes using DPPH, in Khimiya i tekhnologiya rastitel’nykh veshchestv: materialy X Vserossiiskoi nauchnoi konferentsii i shkoly molodykh uchenykh (Chemistry and Technology of Plant Substances: Proceedings of the X All-Russian Scientific Conference and School of Young Scientists), Kazan, 2017, pp. 278–279.
Stolpovskaya, E.V., Trofimova, N.N., and Babkin, V.A., Evaluation of antioxidant activity of dihydroquercetin complexes with biogenic metal ions, Russ. J. Bioorg. Chem., 2017, vol. 43, no. 7, pp. 52–56. https://doi.org/10.1134/S1068162017070160
Trofimova, N.N., Babkin, V.A., and Kiselev, O.I., Complex compounds of zinc and copper(II) ions with dihydroquercetin and their antiviral activity, Izv. Akad. Nauk, Ser. Khim., 2015, no. 6, pp. 1430–1436.
Kostyro, Ya.A., Gogol’, E.S., Lepekhova, S.A., Gol’dberg, O.A., Trofimova, N.N., Stolpovskaya, E.V., and Babkin, V.A., Means for the treatment of wounds and burns, RF Patent no. 2 637 440, Byull. Izobret., 2017, no. 34.
Babkin, V.A., Ostroukhova, L.A., and Trofimova, N.N., Biomassa listvennitsy: ot khimicheskogo sostava do innovatsionnykh produktov (Larch Biomass: From Chemical Composition to Innovative Products), Novosibirsk, 2011.
Panchenko, L.F., Maev, I.V., and Gurevich, K.G., Klinicheskaya biokhimiya mikroelementov (Clinical Biochemistry of Microelements), Moscow, 2004.
Aizikovich, I.V., Aizikovich, B.I., Antonov, A.R., Elovskii, A.A., and Ustinov, D.V., The role of biometals in the pathogenesis of infertility, Vestn. NGU. Ser.: Biol., Klin. Med., 2010, vol. 8, no. 4, pp. 171–177.
Korochkina, E.A., The influence of trace elements zinc, cobalt, iodine, selenium, manganese, copper on the health and productive qualities of animals, Genet. Razv. Zhiv., 2016, no. 3, pp. 69–73.
Filippova, V.A. and Lysenkova, A.V., Chemistry of biogenic elements (lecture), Probl. Zdor. Ekol., 2013, no. 4 (38), pp. 72–78.
Rustembekova, S.A., Ametov, A.S., and Tliashinova, A.M., Elemental imbalance in thyroid pathology, Russ. Med. Zh. Endokrinol., 2008, vol. 16, no. 16, pp. 1078–1081.
Agadzhanyan, N.A., Skal’nyi, A.V., and Detkov, V.Yu., Elemental portrait of a person: Morbidity, demography and the problem of managing the health of the nation, Ekol. Chel., 2013, no. 11, pp. 3–12.
Bakhtina, G.G., Len’ko, O.A., and Sukhanova, S.E., Human microelementosis and ways to correct their deficiency, Patol. Krovoobr. Kardiokhir., 2007, no. 4, pp. 82–89.
Loiko, O.P., Mauletova, R.M., Mashentseva, A.A., Khalitova, A.I., and Tuleuov, B.I., Synthesis and study of biological activity of complex compounds of quercetin with some d metals, in I Mezhdunarodnaya Rossiisko-Kazakhstanskaya konferentsiya po khimii i khimicheskoi tekhnologii (I International Russian–Kazakhstan Conference on Chemistry and Chemical Technology), Tomsk, 2011, pp. 313–316.
Bravo, A. and Anacona, J.R., Metal complexes of the flavonoid quercetin: antibacterial properties, Transit. Metal Chem., 2001, vol. 26, pp. 20–23.
Satterfield, M. and Brodbelt, J.S., Enhanced detection of flavonoids by metal complexation and electrospray ionization mass spectrometry, Anal. Chem., 2000, vol. 72, no. 24, pp. 5898–5906.
Satterfield, M. and Brodbelt, J.S., Structural characterization of flavonoid glycosides by collisionally activated dissociation of metal complexes, J. Am. Soc. Mass Spectrom., 2001, vol. 12, pp. 537–549.
Pikulski, M. and Brodbelt, J.S., Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry, J. Am. Soc. Mass Spectrom., 2003, vol. 14, pp. 1437–1453. https://doi.org/10.1016/j.jasms.2003.07.002
Pikulski, M., Wilson, J.J., Aguilar, A., and Brodbelt, J.S., Amplification of infrared multiphoton dissociation efficiency in a quadruple ion trap using IR-active ligands, Anal. Chem., 2006, vol. 78, no. 24, pp. 8512–8517. https://doi.org/10.1021/ac061472k
Pikulski, M., Aguilar, A., and Brodbelt, J.S., Tunable transition metal–ligand complexation for enhanced elucidation of flavonoid diglycosides by electrospray ionization mass spectrometry, J. Am. Soc. Mass Spectrom., 2007, vol. 18, pp. 422–431. https://doi.org/10.1016/j.jasms.2006.10.011
Davis, D.D. and Brodbelt, J.S., Determination of the glycosylation site of flavonoid monoglucosides by metal complexation and tandem mass spectrometry, J. Am. Soc. Mass Spectrom., 2004, vol. 15, pp. 1287–1299. https://doi.org/10.1016/j.jasms.2004.06.003
Babkin, V.A., Ostroukhova, L.A., Babkin, D.V., and Malkov, Yu.A., A method for obtaining dihydroquercetin, RF Patent no. 2 158 598, 2000.
Gosudarstvennaya farmakopeya OFS 1.2.1.0005.15. (State Pharmacopoeia, Official Pharmacopeia Article 1.2.1.0005.15), 13th ed.
De Souza, R.F.V. and De Giovani, W.F., Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids, Spectrochim. Acta Part A., 2005, vol. 61, pp. 1985–1990. https://doi.org/10.1016/j.saa.2004.07.029
Cornard, J.P. and Merlin, J.C., Spectroscopic and structural study of complexes of quercetin with Al (III), J. Inorg. Biochem., 2002, vol. 92, pp. 19–27.
Zheltoukhova, E.P., Koval’chukova, O.V., Zaitsev, B.E., and Strashnova, S.B., Complex compounds of certain metals with quercetin, in Novye dostizheniya v khimii i khimicheskoi tekhnologii rastitel’nogo syr’ya: materialy IV Vserossiiskoi konferentsii (New Advances in Chemistry and Chemical Technology of Plant Materials: Proceedings of the IV All-Russian Conference), Barnaul, 2009, vol. 2, pp. 217–218.
Torreggiani, A., Tamba, M., Trinchero, A., and Bonora, S., Copper(II)–Wuercetin complexes in aqueous solutions: spectroscopic and kinetic properties, J. Mol. Struct., 2005, vol. 744–747, pp. 759–766. https://doi.org/10.1016/j.molstruc.2004.11.081
Cornard, J.P., Boudet, A.C., and Merlin, J.C., Complexes of Al(III) with 3′4′-dihydroxiflavone: Characterization, theoretical and spectroscopic study, Spectrochim. Acta Part A, 2001, vol. 57, no. 3, pp. 591–602.
Chervyakovskii, E.M., Kurchenko, V.P., and Kostyuk, V.A., Role of flavonoids in biological reactions with electron transfer, Tr. Beloruss. Gos. Univ., 2009, vol. 4, part 1, pp. 9–26.
Heim, K.E., Tagliaferro, A.R., and Bobilya, D.J., Flavonoid antioxidants: Chemistry, metabolism and structure–activity relationships, J. Nutr. Biochem., 2002, vol. 13, pp. 572–584.
Panhwar, Q.K. and Memon, Sh., Synthesis, characterization and antioxidant study of Tin(II)-rutin complex: Exploration of tin packaging hazards, Inorg. Chim. Acta, 2013, vol. 407, pp. 252–260. https://doi.org/10.1016/j.ica.2013.08.001
Shchekatikhina, A.S. and Kurchenko, V.P., Spectrophotometric characteristics of complexes of quercetin, morin, taxifolin, and silibinin with copper(II) ions, Tr. Beloruss. Gos. Univ., 2011, vol. 6, part 1, pp. 76–85.
Zenkevich, I.G., Yeshchenko, Yu.A., Makarov, V.G., Kolesnik, Yu.A., Shmatkov, D.A., Tikhonov, V.P., and Tashlitskiy, V.M., Comparative characteristics of the properties and stereoisomerism of dihydroquercetin. Composition of the flavonoid complex of larch, in Aktual’nye problemy sozdaniya novykh lekarstvennykh preparatov prirodnogo proiskhozhdeniya. Fitofarm-2006: materialy X mezhdunarodnogo s”ezda (Actual Problems of Creating New Drugs of Natural Origin. Phytopharm 2006: Proceedings of the 10th International Congress), St. Petersburg, 2006, pp. 93–109.
Vyaznikova, M.Yu., Nikolaeva, S.S., Smirnova, L.P., and Bykov, V.A., Study of bound water in quercetin, Khim.-Farm. Zh., 1997, vol. 31, no. 2, pp. 39–41.
Vyaznikova, M.Yu., Nikolayeva, S.S., Bykov, V.A., Yakovleva, L.V., Rulenko, I.A., Tyukavkina, N.A., and Kolesnik, Yu.A., Investigation of the state of water in a standard sample of dihydroquercetin and in a new phytopreparation dikvertin, Khim.-Farm. Zh., 1997, vol. 31, no. 2, pp. 42–45.
Selifonova, E.I., Chernova, R.K., and Koblova, O.E., Thermogravimetric study of L-α-amino acids Izv. Sarat. Univ., Nov. Ser., Ser.: Khim. Biol. Ekol., 2008, vol. 8, no. 2, pp. 23–28.
Tarasevich, B.N., IK spektry osnovnykh klassov organicheskikh soedinenii. Spravochnye materialy (IR Spectra of the Main Classes of Organic Compounds. Reference Materials), Moscow, 2012.
ACKNOWLEDGMENTS
The study was performed with the use of the facilities of the Baikal Analytical Center of Collective Use (Siberian Branch, Russian Academy of Sciences).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The work does not involve experiments on animals or humans.
Conflict of Interests
Authors declare they have no conflicts of interests.
Additional information
Translated by S. Sidorova
Rights and permissions
About this article
Cite this article
Stolpovskaya, E.V., Trofimova, N.N., Babkin, V.A. et al. A Study and Optimization of Complexation of Cobalt Ions with Dihydroquercetin in Aqueous Solutions. Russ J Bioorg Chem 46, 1351–1357 (2020). https://doi.org/10.1134/S1068162020070158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020070158