Abstract
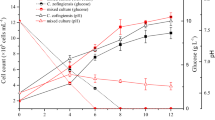
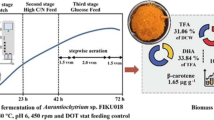
A marine organism, belonging to the Thraustochytrids family was isolated from mangroves of Mumbai, India. The isolated strain was identified as Aurantiochytrium limacinum by internal transcribed spacer sequence analysis. Optimization of process parameters yielded 14.47 g/L dry cell weight containing 55–58% oil in 3.5 days’ cultivation on glucose, yeast extract, and peptone in the bioreactor. Docosahexaenoic acid (DHA) was found to be the dominant long-chain polyunsaturated fatty acid, accounting for 32–35% of total fatty acid content. The process parameter was tweaked to simultaneously synthesize astaxanthin along with DHA. The concurrent synthesis of DHA and astaxanthin-containing biomass establishes the isolated strain as a perfect choice for aquafeed.
Accession number: NCBI accession number MN046792.








Similar content being viewed by others
References
Aki T, Hachida K, Yoshinaga M, Katai Y, Yamasaki T, Kawamoto S, Ono K (2003) Thraustochytrid as a potential source of carotenoids. J Am Oil ChemSoc 80(8):789
Armenta RE, Burja A, Radianingtyas H, Barrow CJ (2006) Critical assessment of various techniques for the extraction of carotenoids and co-enzyme Q10 from the Thraustochytrid strain ONC-T18. J Agric Food Chem 54(26):9752–9758
Bajpai PK, Bajpai P, Ward OP (1991) Optimization of production of docosahexaenoic acid (DHA) by Thraustochytrium aureum ATCC 34304. J Am Oil ChemSoc 68(7):509–514
Barclay WR, Meager KM, Abril JR (1994) Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J ApplPhycol 6(2):123–129
Berryman KT (2012) Isolation of marine protists for production of polyunsaturated fatty acids. https://docplayer.net/86481864-Kevin-thomas-berryman-submitted-in-partial-fulfilment-of-the-requirements-for-the-degree-of-master-of-science.html#show_full_text. Accessed 11 Jan 2021
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem 37(8):911–917
Bongiorni L, Jain R, Raghukumar S, Aggarwal RK (2005) Thraustochytrium gaertnerium sp. nov.: a new thraustochytridstramenopilanprotist from mangroves of Goa, India. Protist 156(3):303–315
Bowles RD, Hunt AE, Bremer GB, Duchars MG, Eaton RA (1999) Long-chain n-3 polyunsaturated fatty acid production by members of the marine protistan group the thraustochytrids: screening of isolates and optimization of docosahexaenoic acid production. J Biotechnol 70:193–202
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. ApplMicrobiolBiotechnol 72(6):1161–1169
Carlson SE, Colombo J (2016) Docosahexaenoic acid and arachidonic acid nutrition in early development. AdvPediatr 63(1):453
Chen G, Fan KW, Lu FP, Li Q, Aki T, Chen F, Jiang Y (2010) Optimization of nitrogen source for enhanced production of squalene from thraustochytridAurantiochytrium sp. N Biotechnol 27:382–389
Cooksey KE, Guckert JB, Williams SA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6(6):333–345
Das UN (2003) Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 19(1):62–65
De Swaaf M, Pronk J, Sijtsma L (2003a) Fed-batch cultivation of the docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. ApplMicrobiolBiotechnol 61(1):40–43
De Swaaf ME, Sijtsma L, Pronk JT (2003b) High cell density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. BiotechnolBioeng 81(6):666–672
Fan KW, Chen F, Jones EB, Vrijmoed LL (2001) Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J IndMicrobiolBiot 27(4):199–202
Goldstein S (1963) Development and nutrition of new species of Thraustochytrium. Am J Bot 50(3):271–279
Gong Y, Liu J, Jiang M, Liang Z, Jin H, Hu X, Hu C (2015) Improvement of omega-3 docosahexaenoic acid production by marine dinoflagellateCrypthecodinium cohnii using rapeseed meal hydrolysate and waste molasses as feedstock. PLoS ONE 10(5):e0125368
Gupta A, Barrow CJ, Puri M (2012) Omega-3 biotechnology: thraustochytrids as a novel source of omega-3 oils. BiotechnolAdv 30(6):1733–1745
Gupta A, Wilkens S, Adcock JL, Puri M, Barrow CJ (2013) Pollen baiting facilitates the isolation of marine thraustochytrids with potential in omega-3 and biodiesel production. J IndMicrobiolBiot 40(11):1231–1240
Hauvermale A, Kuner J, Rosenzweig B, Guerra D, Diltz S, Metz JG (2006) Fatty acid production in Schizochytrium sp.: involvement 2 of a polyunsaturated fatty acid synthase and a type I fatty acid synthase. Lipids 41:739–747
Jakobsen AN (2008) Compatible solutes and docosahexaenoic acid accumulation of thraustochytrids of the Aurantiochytrium group
Jaseera K, Kaladharan P (2019) Thrauastochytrids in aquaculture—can it replace fish meal in aquaculture? AquacSpectr 2:25–27
Kumari S, Vira C, Lali AM, Prakash G (2020) Heterologous expression of a mutant Orange gene from Brassica oleracea increases carotenoids and induces phenotypic changes in the microalga Chlamydomonas reinhardtii. Algal Res 47:101871
Lali AM, Nagwekar PD, Varavadekar JS, Wadekar PC, Gujarathi SS, Valte RD, Birhade SH, Odaneth AA, Inventors (2012) Chemical. Engr. Department. Inst. of Chemical Tech. (Deemed Univ.), assignee. Method for production of fermentable sugars from biomass. United States patent US 8,338,139
Lee Chang KJ, Paul H, Nichols PD, Koutoulis A, Blackburn SI (2015) Australian thraustochytrids: potential production of dietary long-chain omega-3 oils using crude glycerol. J Funct Foods 19:810–820
Lippmeier JC, Crawford KS, Owen CB, Rivas AA, Metz JG, Apt KE (2009) Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids 44:621–630
Mendes A, Reis A, Vasconcelos R, Guerra P, da Silva TL (2009) Crypthecodinium cohnii with emphasis on DHA production: a review. J ApplPhycol 21(2):199–214
Mo C, Douek J, Rinkevich B (2002) Development of a PCR strategy for thraustochytrid identification based on 18S rDNA sequence. Mar Biol 140(5):883–889
Mohebi-Nejad A, Bikdeli B (2014) Omega-3 supplements and cardiovascular diseases. Tanaffos 13(1):6
Park H, Kwak M, Seo J, Ju J, Heo S, Park S, Hong W (2018) Enhanced production of carotenoids using a thraustochytridmicroalgal strain containing high levels of docosahexaenoic acid-rich oil. BioprocBiosystEng 41(9):1355–1370
Perveen Z, Ando H, Ueno A, Ito Y, Yamamoto Y, Yamada Y, Takagi T, Kaneko T, Kogame K, Okuyama H (2006) Isolation and characterization of a novel thraustochytrid-like microorganism that efficiently produces docosahexaenoic acid. BiotechnolLett 28(3):197–202
Raghukumar S (2008) Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol 10(6):631–640
Rathod JP, Vira C, Lali AM, Prakash G (2020) Metabolic engineering of Chlamydomonas reinhardtii for enhanced β-carotene and lutein production. Appl. Biochem Biotech 190(4):1457–1469
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86(11):807–815
Sarnaik A, Nambissan V, Pandit R, Lali A (2018) Recombinant Synechococcuselongatus PCC 7942 for improved zeaxanthin production under natural light conditions. Algal Res 36:139–151
Singh A, Ward OP (1996) Production of high yields of docosahexaenoic acid by Thraustochytrium roseum ATCC 28210. J IndMicrobiol 16(6):370–373
Siriwardhana N, Kalupahana NS, Moustaid-Moussa N (2012) Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res 65:211–222
Velmurugan N, Sathishkumar Y, Yim SS, Lee YS, Park MS, Yang JW, Jeong KJ (2014) Study of cellular development and intracellular lipid bodies’ accumulation in the thraustochytridAurantiochytrium sp. KRS101. BioresourTechnol 161:149–154
Watanabe K, Arafiles KHV, Higashi R, Okamura Y, Tajima T, Matsumura Y, Nakashimada Y, Matsuyama K, Aki T (2018) Isolation of high carotenoid-producing Aurantiochytrium sp. mutants and improvement of astaxanthin productivity using metabolic information. J Oleo Sci 67(5):571–578
Yang ST (ed) (2011) Bioprocessing for value-added products from renewable resources: new technologies and applications. Elsevier, Amsterdam
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. ApplMicrobiolBiotechnol 49(1):72–76
Author information
Authors and Affiliations
Contributions
Experiments were planned by GP and AML. Isolation work was executed by SV. SK planned and executed the strain identification studies. PRP did the growth optimization experiments. Data were analysed by PRP, SK, AML and GP. The manuscript is written by PRP and GP. Funding for the work was facilitated by AML.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pawar, P.R., Velani, S., Kumari, S. et al. Isolation and optimization of a novel thraustochytrid strain for DHA rich and astaxanthin comprising biomass as aquafeed supplement. 3 Biotech 11, 71 (2021). https://doi.org/10.1007/s13205-020-02616-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02616-4