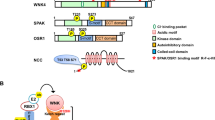
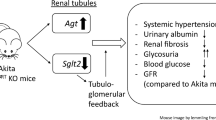
Abstract
Familial hyperkalemic hypertension (FHHt; also called pseudohypoaldosteronism type II) is a hereditary hypertensive disease which can be caused by mutations in four genes: WNK1 [with no lysine (K) 1], WNK4, Kelch-like3 (KLHL3), and cullin3 (CUL3). Decreased KLHL3 expression was identified as being involved in the pathogenesis of FHHt caused by cullin 3 disease mutations. Recent studies have revealed an increased WNK4 and hence Na-Cl cotransporter (NCC) activity in the db/db mice, resulting from PKC-mediated KLHL3 phosphorylation, which impairs the degradation of its substrate, WNK4. However, whether WNK4 and NCC were activated in type 1 diabetes still remains unclear. We created streptozotocin-induced type 1 diabetic mice and revealed that renal WNK-oxidative stress response kinase-1/STE20/SPS1-related proline alanine–rich kinase (OSR1/SPAK)-NCC cascade was activated, whereas KLHL3 expression was markedly decreased and CUL3 was heavily neddylated. Moreover, decreased KLHL3 was reversed and WNK1 and WNK4 abundance increased by MLN4924, a neddylation inhibitor. In vitro, our study also showed decreased KLHL3 abundance without any significant change in phosphorylated KLHL3 under high glucose exposure. These results indicate that decreased KLHL3 likely plays a role in the pathogenesis of renal sodium reabsorption in hyperglycemic conditions.






Similar content being viewed by others
Abbreviations
- FHHt:
-
Familial hyperkalemic hypertension
- KLHL3:
-
Kelch-like3
- CUL3:
-
Cullin3
- GLUT:
-
glucose transporter
- NCC:
-
Na-Cl cotransporter
- PKC:
-
Protein kinase C
- PKA:
-
Protein kinase A
- NKCC:
-
Na–K–Cl cotransporter
- T2DM:
-
type 2 diabetes mellitus
- PM:
-
Plasma membrane
- OSR1:
-
Oxidative stress response kinase-1
- SPAK:
-
STE20/SPS1-related proline alanine–rich kinase
- TBC1D:
-
Tre-2-BUB2-CDC16 domain family member
- WNK kinase:
-
With-no-lysine protein kinase
- T1DM:
-
Type 1 diabetes mellitus
- HCTZ:
-
Hydrochlorothiazide
- HG:
-
High glucose
- LG:
-
Low glucose
- LM:
-
Low mannitol
References
Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA (2001) Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol 281:F639–F648. https://doi.org/10.1152/ajprenal.2001.281.4.F639
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102. https://doi.org/10.1038/nature10814
Boyd-Shiwarski CR, Shiwarski DJ, Roy A, Namboodiri HN, Nkashama LJ, Xie J, McClain KL, Marciszyn A, Kleyman TR, Tan RJ, Stolz DB, Puthenveedu MA, Huang CL, Subramanya AR (2018) Potassium-regulated distal tubule WNK bodies are kidney-specific WNK1 dependent. Mol Biol Cell 29:499–509. https://doi.org/10.1091/mbc.E17-08-0529
Chavez-Canales M, Arroyo JP, Ko B, Vazquez N, Bautista R, Castaneda-Bueno M, Bobadilla NA, Hoover RS, Gamba G (2013) Insulin increases the functional activity of the renal NaCl cotransporter. J Hypertens 31:303–311. https://doi.org/10.1097/HJH.0b013e32835bbb83
Chen P, Hu T, Liang Y, Jiang Y, Pan Y, Li C, Zhang P, Wei D, Li P, Jeong LS, Chu Y, Qi H, Yang M, Hoffman RM, Dong Z, Jia L (2015) Synergistic inhibition of autophagy and neddylation pathways as a novel therapeutic approach for targeting liver cancer. Oncotarget 6:9002–9017. https://doi.org/10.18632/oncotarget.3282
Cornelius RJ, Si J, Cuevas CA, Nelson JW, Gratreak BDK, Pardi R, Yang CL, Ellison DH (2018) Renal COP9 signalosome deficiency alters CUL3-KLHL3-WNK Signaling Pathway. J Am Soc Nephrol 29:2627–2640. https://doi.org/10.1681/asn.2018030333
de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B, Eckel RH (2014) Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 37:2843–2863. https://doi.org/10.2337/dc14-1720
Downie ML, Ulrich EH, Noone DG (2018) An update on hypertension in children with type 1 diabetes. Can J Diabetes 42:199–204. https://doi.org/10.1016/j.jcjd.2018.02.008
Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA (2008) Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134:995–1006. https://doi.org/10.1016/j.cell.2008.07.022
Fidler TP, Marti A, Gerth K, Middleton EA, Campbell RA, Rondina MT, Weyrich AS, Abel ED (2019) Glucose metabolism is required for platelet hyperactivation in a murine model of type 1 diabetes. Diabetes 68:932–938. https://doi.org/10.2337/db18-0981
Gordon RD (1986) Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension 8:93–102. https://doi.org/10.1161/01.hyp.8.2.93
Hadchouel J, Ellison DH, Gamba G (2016) Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78:367–389. https://doi.org/10.1146/annurev-physiol-021115-105431
Han S, Shin H, Oh JW, Oh YJ, Her NG, Nam DH (2019) The protein neddylation inhibitor MLN4924 suppresses patient-derived glioblastoma cells via inhibition of ERK and AKT signaling. Cancers 11. https://doi.org/10.3390/cancers11121849
Hao R, Song Y, Li R, Wu Y, Yang X, Li X, Qian F, Ye RD, Sun L (2019) MLN4924 protects against interleukin-17A-induced pulmonary inflammation by disrupting ACT1-mediated signaling. Am J Physiol Lung Cell Mol Physiol 316:L1070–l1080. https://doi.org/10.1152/ajplung.00349.2018
Ishizawa K, Wang Q, Li J, Xu N, Nemoto Y, Morimoto C, Fujii W, Tamura Y, Fujigaki Y, Tsukamoto K, Fujita T, Uchida S, Shibata S (2019) Inhibition of sodium glucose cotransporter 2 attenuates the dysregulation of Kelch-like 3 and NaCl cotransporter in obese diabetic mice. J Am Soc Nephrol 30:782–794. https://doi.org/10.1681/asn.2018070703
Ishizawa K, Wang Q, Li J, Yamazaki O, Tamura Y, Fujigaki Y, Uchida S, Lifton RP, Shibata S (2019) Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc Natl Acad Sci U S A 116:3155–3160. https://doi.org/10.1073/pnas.1817281116
Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, Ellison DH (2012) Enhanced phosphorylation of Na(+)-Cl- co-transporter in experimental metabolic syndrome: role of insulin. Clin Sci (Lond) 123:635–647. https://doi.org/10.1042/cs20120003
Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson J, Ehrlich J, Garg S, Eckel RH, Rewers MJ (2005) Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care 28:301–306. https://doi.org/10.2337/diacare.28.2.301
McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH (2014) Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124:4723–4736. https://doi.org/10.1172/jci76126
Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H (2005) WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280:42685–42693. https://doi.org/10.1074/jbc.M510042200
Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, Fan GC, Lu Y, Abel ED, Greer PA, Peng T (2016) Mitochondrial calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: a novel mechanism contributing to diabetic cardiomyopathy. Diabetes 65:255–268. https://doi.org/10.2337/db15-0963
Nishida H, Sohara E, Nomura N, Chiga M, Alessi DR, Rai T, Sasaki S, Uchida S (2012) Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension 60:981–990. https://doi.org/10.1161/hypertensionaha.112.201509
Ohh M, Kim WY, Moslehi JJ, Chen Y, Chau V, Read MA, Kaelin WG Jr (2002) An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep 3:177–182. https://doi.org/10.1093/embo-reports/kvf028
Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA (2010) Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78:160–169. https://doi.org/10.1038/ki.2010.130
Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH (2009) Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119:2601–2612. https://doi.org/10.1172/jci38323
Sasaki E, Susa K, Mori T, Isobe K, Araki Y, Inoue Y, Yoshizaki Y, Ando F, Mori Y, Mandai S, Zeniya M, Takahashi D, Nomura N, Rai T, Uchida S, Sohara E (2017) KLHL3 knockout mice reveal the physiological role of KLHL3 and the pathophysiology of pseudohypoaldosteronism type II caused by mutant KLHL3. Mol Cell Biol 37. https://doi.org/10.1128/mcb.00508-16
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP (2013) Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A 110:7838–7843. https://doi.org/10.1073/pnas.1304592110
Shibata S, Arroyo JP, Castaneda-Bueno M, Puthumana J, Zhang J, Uchida S, Stone KL, Lam TT, Lifton RP (2014) Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci U S A 111:15556–15561. https://doi.org/10.1073/pnas.1418342111
Sohara E, Uchida S (2016) Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder. Nephrol Dial Transplant 31:1417–1424. https://doi.org/10.1093/ndt/gfv259
Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S (2014) Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet 23:5052–5060. https://doi.org/10.1093/hmg/ddu217
Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH (2015) Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21:39–50. https://doi.org/10.1016/j.cmet.2014.12.006
Thomson MN, Schneider W, Mutig K, Ellison DH, Kettritz R, Bachmann S (2019) Patients with hypokalemia develop WNK bodies in the distal convoluted tubule of the kidney. Am J Physiol Ren Physiol 316:F292–f300. https://doi.org/10.1152/ajprenal.00464.2018
Thomson MN, Cuevas CA, Bewarder TM, Dittmayer C, Miller LN, Si J, Cornelius RJ, Su XT, Yang CL, McCormick JA, Hadchouel J, Ellison DH, Bachmann S, Mutig K (2020) WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia. Am J Physiol Ren Physiol 318:F216–f228. https://doi.org/10.1152/ajprenal.00232.2019
Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S (2013) Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep 3:858–868. https://doi.org/10.1016/j.celrep.2013.02.024
Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, McCormick JA, Yang CL, Wang WH, Ellison DH (2018) Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93:893–902. https://doi.org/10.1016/j.kint.2017.10.023
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science (New York, NY) 293:1107–1112. https://doi.org/10.1126/science.1062844
Yang CL, Angell J, Mitchell R, Ellison DH (2003) WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111:1039–1045. https://doi.org/10.1172/jci17443
Yang SS, Fang YW, Tseng MH, Chu PY, Yu IS, Wu HC, Lin SW, Chau T, Uchida S, Sasaki S, Lin YF, Sytwu HK, Lin SH (2013) Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24:1587–1597. https://doi.org/10.1681/asn.2012070742
Yoshida S, Araki Y, Mori T, Sasaki E, Kasagi Y, Isobe K, Susa K, Inoue Y, Bomont P, Okado T, Rai T, Uchida S, Sohara E (2018) Decreased KLHL3 expression is involved in the pathogenesis of pseudohypoaldosteronism type II caused by cullin 3 mutation in vivo. Clin Exp Nephrol 22:1251–1257. https://doi.org/10.1007/s10157-018-1593-z
Yoshizaki Y, Mori Y, Tsuzaki Y, Mori T, Nomura N, Wakabayashi M, Takahashi D, Zeniya M, Kikuchi E, Araki Y, Ando F, Isobe K, Nishida H, Ohta A, Susa K, Inoue Y, Chiga M, Rai T, Sasaki S, Uchida S, Sohara E (2015) Impaired degradation of WNK by Akt and PKA phosphorylation of KLHL3. Biochem Biophys Res Commun 467:229–234. https://doi.org/10.1016/j.bbrc.2015.09.184
Zhou Q, Sun Y (2019) MLN4924: additional activities beyond neddylation inhibition. Mol Cell Oncol 6:e1618174. https://doi.org/10.1080/23723556.2019.1618174
Acknowledgments
We thank Chao-Ling Yang and David H. Ellison from Oregon Health and Science University for providing antibodies used in this study.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No 81770706 and 81570634).
Author information
Authors and Affiliations
Contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, Q., Zhang, Y., Jiang, GR. et al. Decreased KLHL3 expression is involved in the activation of WNK-OSR1/SPAK-NCC cascade in type 1 diabetic mice. Pflugers Arch - Eur J Physiol 473, 185–196 (2021). https://doi.org/10.1007/s00424-020-02509-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02509-8