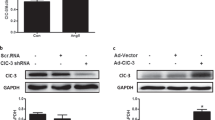
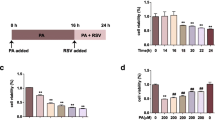
Abstract
Chloride intracellular channel 1 (CLIC1) is a sensor of oxidative stress in endothelial cells (EC). However, the mechanism by which CLIC1 mediate the regulation of endothelial dysfunction has not been established. In this study, overexpressed CLIC1 impaired the ability of the vascular cells to resist oxidative damage and promoted cellular senescence. Besides, suppressed CLIC1 protected against cellular senescence and dysfunction in Human Umbilical Vein Endothelial Cells (HUVECs) through the Nrf2/HO-1 pathway. We also found that ROS-activated CLIC1-induced oxidative stress in HUVECs. Nrf2 nuclear translocation was inhibited by CLIC1 overexpression, but was enhanced by IAA94 (CLICs inhibitor) treatment or knockdown of CLIC1. The Nrf2/HO-1 pathway plays a critical role in the anti-oxidative effect of suppressing CLIC1. And inhibition of CLIC1 decreases oxidative stress injury by downregulating the levels of ROS, MDA, and the expression of EC effectors (ICAM1 and VCAM1) protein expression and promotes the activity of superoxide dismutase (SOD). The AMPK-mediated signaling pathway activates Nrf2 through Nrf2 phosphorylation and nuclear translocation, which is also regulated by CLIC1. Moreover, the activation of CLIC1 contributes to H2O2-induced mitochondrial dysfunction and activation of mitochondrial fission. Therefore, elucidation of the mechanisms by which CLIC1 is involved in these pivotal pathways may uncover its therapeutic potential in alleviating ECs oxidative stress and age-related cardiovascular disease development.










Similar content being viewed by others
References
Ulmasov, B., Bruno, J., Woost, P. G., & Edwards, J. C. (2007). Tissue and subcellular distribution of CLIC1. BMC Cell Biology, 8, 8. https://doi.org/10.1186/1471-2121-8-8.
Uretmen Kagiali, Z. C., Saner, N., Akdag, M., Sanal, E., Degirmenci, B. S., Mollaoglu, G., & Ozlu, N. (2020). CLIC4 and CLIC1 bridge plasma membrane and cortical actin network for a successful cytokinesis. Life Science Alliance, 3(2), e201900558. https://doi.org/10.26508/lsa.201900558.
Argenzio, E., & Moolenaar, W. H. (2016). Emerging biological roles of Cl- intracellular channel proteins. Journal of Cell Science, 129(22), 4165–4174. https://doi.org/10.1242/jcs.189795.
Littler, D. R., Harrop, S. J., Goodchild, S. C., Phang, J. M., Mynott, A. V., Jiang, L., & Curmi, P. M. G. (2010). The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Letters, 584(10), 2093–2101. https://doi.org/10.1016/j.febslet.2010.01.027.
Al Khamici, H., Brown, L. J., Hossain, K. R., Hudson, A. L., Sinclair-Burton, A. A., Ng, J. P. M., & Valenzuela, S. M. (2015). Members of the Chloride Intracellular Ion Channel Protein Family Demonstrate Glutaredoxin-Like Enzymatic Activity. PLoS ONE, 10(1), e115699. https://doi.org/10.1371/journal.pone.0115699.
Littler, D. R., Harrop, S. J., Fairlie, W. D., Brown, L. J., Pankhurst, G. J., Pankhurst, S., & Curmi, P. M. G. (2004). The Intracellular Chloride Ion Channel Protein CLIC1 Undergoes a Redox-controlled Structural Transition. Journal of Biological Chemistry, 279(10), 9298–9305. https://doi.org/10.1074/jbc.M308444200.
Tulk, B. M., Kapadia, S., & Edwards, J. C. (2002). CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. American Journal of Physiology-Cell Physiology, 282(5), C1103–C1112. https://doi.org/10.1152/ajpcell.00402.2001.
Singh, H., & Ashley, R. H. (2006). Redox Regulation of CLIC1 by Cysteine Residues Associated with the Putative Channel Pore. Biophysical Journal, 90(5), 1628–1638. https://doi.org/10.1529/biophysj.105.072678.
Rozanski, A., Blumenthal, J. A., & Kaplan, J. (1999). Impact of Psychological Factors on the Pathogenesis of Cardiovascular Disease and Implications for Therapy. Circulation, 99(16), 2192–2217. https://doi.org/10.1161/01.CIR.99.16.2192.
Cai, H., & Harrison, D. G. (2000). Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation Research, 87(10), 840–844. https://doi.org/10.1161/01.RES.87.10.840.
Averaimo, S., Milton, R. H., Duchen, M. R., & Mazzanti, M. (2010). Chloride intracellular channel 1 (CLIC1): sensor and effector during oxidative stress. FEBS Letters, 584(10), 2076–2084. https://doi.org/10.1016/j.febslet.2010.02.073.
Xu, Y., Zhu, J., Hu, X., Wang, C., Lu, D., Gong, C., & Zong, L. (2016). CLIC1 Inhibition Attenuates Vascular Inflammation, Oxidative Stress, and Endothelial Injury. PLOS ONE, 11(11), e0166790 https://doi.org/10.1371/journal.pone.0166790.
Zhu, J., Xu, Y., Ren, G., Hu, X., Wang, C., Yang, Z., & Lu, D. (2017). Tanshinone IIA Sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. European Journal of Pharmacology, 815, 427–436. https://doi.org/10.1016/j.ejphar.2017.09.047.
Nguyen, T., Nioi, P., & Pickett, C. B. (2009). The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. Journal of Biological Chemistry, 284(20), 13291–13295. https://doi.org/10.1074/jbc.R900010200.
Choi, A. M. K., & Alam, J. (1996). Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. American Journal of Respiratory Cell and Molecular Biology, 15(1), 9–19. https://doi.org/10.1165/ajrcmb.15.1.8679227.
Araujo, J. A., Zhang, M., & Yin, F. (2012). Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Frontiers in Pharmacology, 3, 119. https://doi.org/10.3389/fphar.2012.00119.
Biswas, C., Shah, N., Muthu, M., La, P., Fernando, A. P., Sengupta, S., & Dennery, P. A. (2014). Nuclear Heme Oxygenase-1 (HO-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-oxidant Defenses. Journal of Biological Chemistry, 289(39), 26882–26894. https://doi.org/10.1074/jbc.M114.567685.
Wang, C., Le, Y., Lu, D., Zhao, M., Dou, X., & Zhang, Q. (2020). Triphenyl phosphate causes a sexually dimorphic metabolism dysfunction associated with disordered adiponectin receptors in pubertal mice. Journal of Hazardous Materials, 388, 121732. https://doi.org/10.1016/j.jhazmat.2019.121732.
Li, S., & Wang, C. (2018). Study on the potential way of hepatic cytotoxicity of N, N -dimethylformamide. Journal of Biochemical and Molecular Toxicology, 32(9), e22190. https://doi.org/10.1002/jbt.22190.
Tang, T., Lang, X., Xu, C., Wang, X., Gong, T., Yang, Y., & Zhou, R. (2017). CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nature Communications, 8(1), 202. https://doi.org/10.1038/s41467-017-00227-x.
Lin, S.-C., & Hardie, D. G. (2018). AMPK: sensing Glucose as well as Cellular Energy Status. Cell Metabolism, 27(2), 299–313. https://doi.org/10.1016/j.cmet.2017.10.009.
Wang, L., Zhang, S., Cheng, H., Lv, H., Cheng, G., & Ci, X. (2016). Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radical Biology and Medicine, 101, 401–412. https://doi.org/10.1016/j.freeradbiomed.2016.11.009.
Caja, S., & Enríquez, J. A. (2017). Mitochondria in endothelial cells: Sensors and integrators of environmental cues. Redox Biology, 12, 821–827. https://doi.org/10.1016/j.redox.2017.04.021.
Coultas, L., Chawengsaksophak, K., & Rossant, J. (2005). Endothelial cells and VEGF in vascular development. Nature, 438(7070), 937–945. https://doi.org/10.1038/nature04479.
Abdul-Salam, V. B., Russomanno, G., Chien-Nien, C., Mahomed, A. S., Yates, L. A., Wilkins, M. R., & Wojciak-Stothard, B. (2019). CLIC4/Arf6 Pathway. Circulation Research, 124(1), 52–65. https://doi.org/10.1161/CIRCRESAHA.118.313705.
Takano, K., Liu, D., Tarpey, P., Gallant, E., Lam, A., Witham, S., & Dulhunty, A. F. (2012). An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Human Molecular Genetics, 21(20), 4497–4507. https://doi.org/10.1093/hmg/dds292.
Francisco, M. A., Wanggou, S., Fan, J. J., Dong, W., Chen, X., Momin, A., & Huang, X. (2020). Chloride intracellular channel 1 cooperates with potassium channel EAG2 to promote medulloblastoma growth. Journal of Experimental Medicine, 217(5), e20190971 https://doi.org/10.1084/jem.20190971.
Hernandez-Fernaud, J. R., Ruengeler, E., Casazza, A., Neilson, L. J., Pulleine, E., Santi, A., & Zanivan, S. (2017). Secreted CLIC3 drives cancer progression through its glutathione-dependent oxidoreductase activity. Nature Communications, 8(1), 14206. https://doi.org/10.1038/ncomms14206.
Ueno, Y., Ozaki, S., Umakoshi, A., Yano, H., Choudhury, M. E., Abe, N., & Tanaka, J. (2019). Chloride intracellular channel protein 2 in cancer and non-cancer human tissues: relationship with tight junctions. Tissue barriers, 7(1), 1593775. https://doi.org/10.1080/21688370.2019.1593775.
Loboda, A., Damulewicz, M., Pyza, E., Jozkowicz, A., & Dulak, J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life Sciences, 73(17), 3221–3247. https://doi.org/10.1007/s00018-016-2223-0.
Covas, G., Marinho, H. S., Cyrne, L., & Antunes, F. (2013). Activation of Nrf2 by H2O2: de novo synthesis versus nuclear translocation. Methods in Enzymology, 528, 157–171. https://doi.org/10.1016/B978-0-12-405881-1.00009-4.
Saragih, H., Zilian, E., Jaimes, Y., Paine, A., Figueiredo, C., Eiz-Vesper, B., & Immenschuh, S. (2014). PECAM-1-dependent heme oxygenase-1 regulation via an Nrf2-mediated pathway in endothelial cells. Thrombosis and Haemostasis, 111(06), 1077–1088. https://doi.org/10.1160/TH13-11-0923.
Joo, M. S., Kim, W. D., Lee, K. Y., Kim, J. H., Koo, J. H., & Kim, S. G. (2016). AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Molecular and Cellular Biology, 36(14), 1931–1942. https://doi.org/10.1128/MCB.00118-16.
Zhao, H., Li, T., Wang, K., Zhao, F., Chen, J., Xu, G., & Pan, X. (2019). AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nature Cell Biology, 21(4), 476–486. https://doi.org/10.1038/s41556-019-0296-3.
Kongsuphol, P., Cassidy, D., Hieke, B., Treharne, K. J., Schreiber, R., Mehta, A., & Kunzelmann, K. (2009). Mechanistic Insight into Control of CFTR by AMPK. Journal of Biological Chemistry, 284(9), 5645–5653. https://doi.org/10.1074/jbc.M806780200.
King, S. J., Bunz, M., Chappell, A., Scharl, M., Docherty, M., Jung, B., & McCole, D. F. (2019). AMPK mediates inhibition of electrolyte transport and NKCC1 activity by reactive oxygen species. American Journal of Physiology-Gastrointestinal and Liver Physiology, 317(2), G171–G181. https://doi.org/10.1152/ajpgi.00317.2018.
Liu, B., Billington, C. K., Henry, A. P., Bhaker, S. K., Kheirallah, A. K., Swan, C., & Hall, I. P. (2018). Chloride intracellular channel 1 (CLIC1) contributes to modulation of cyclic AMP-activated whole-cell chloride currents in human bronchial epithelial cells. Physiological Reports, 6(2), e13508. https://doi.org/10.14814/phy2.13508.
Kim, Y.-M., Youn, S.-W., Sudhahar, V., Das, A., Chandhri, R., Cuervo Grajal, H., & Ushio-Fukai, M. (2018). Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Reports, 23(12), 3565–3578. https://doi.org/10.1016/j.celrep.2018.05.054.
van der Bliek, A. M., Shen, Q., & Kawajiri, S. (2013). Mechanisms of Mitochondrial Fission and Fusion. Cold Spring Harbor Perspectives in Biology, 5(6), a011072–a011072. https://doi.org/10.1101/cshperspect.a011072.
Ponnalagu, D., Gururaja Rao, S., Farber, J., Xin, W., Hussain, A. T., Shah, K., & Singh, H. (2016). Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion, 27, 6–14. https://doi.org/10.1016/j.mito.2016.01.001.
Cheng, Q., Wan, Y., Yang, W., Tian, M., Wang, Y., He, H., & Liu, X. (2020). Gastrodin protects H9c2 cardiomyocytes against oxidative injury by ameliorating imbalanced mitochondrial dynamics and mitochondrial dysfunction. Acta Pharmacologica Sinica, 41(10), 1314–1327. https://doi.org/10.1038/s41401-020-0382-x.
Acknowledgements
Thanks for all the contributors and participants.
Funding
The authors would like to thank National Natural Science Foundation of China (No. 82074057), the Key Laboratory of Integrative Chinese and Western Medicine for the Diagnosis and Treatment of Circulatory Diseases of Zhejiang Province (No. 2C32005), Zhejiang Provincial Natural Science Foundation of China (No. LY19H280003, No. LY20H280007), and Zhejiang Univ. Students Science and Technology Innovation Activity Plan Project (No. 2019R410037) for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, D., Le, Y., Ding, J. et al. CLIC1 Inhibition Protects Against Cellular Senescence and Endothelial Dysfunction Via the Nrf2/HO-1 Pathway. Cell Biochem Biophys 79, 239–252 (2021). https://doi.org/10.1007/s12013-020-00959-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-020-00959-6