Abstract
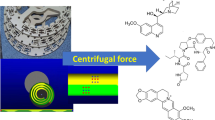
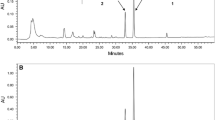
A new dimeric aporphine alkaloid, biscocsarmine, 2, together with lirioferine, 1, and cocsarmine, 3, were isolated from the ethanol extract of the stems of Triclisia dictyophylla Diels (Menispermaceae) using a preparative pH-zone refining CCC in a single step. The first solvent system family tested for this fractionation was hexane–ethyl acetate–methanol–water (HEMWat) at a series of solvent ratios, and hydrochloric acid and triethylamine at 5 mM as eluter/retainer. None of these HEMWat ratios, however, gave suitable K for the target alkaloids. Ethyl acetate–n-butanol–water (EBuWat) was tested instead and the selected solvent system was composed of ethyl acetate–n-butanol–water 5:5:10 (v/v), where triethylamine (60 mM) was added to the upper organic stationary phase as a retainer and hydrochloric acid (5 mM) to the aqueous mobile phase as an eluter. The chemical structures of the isolated compounds were elucidated based on comprehensive spectroscopic and spectrometric techniques. This is the first report of aporphine alkaloids in this plant species.




Similar content being viewed by others
Abbreviations
- EBuWat:
-
Ethyl acetate–n-butanol–water
- HEMWa:
-
Hexane–ethyl acetate–methanol–water
- TEA:
-
Trimethylamine
References
Troupin G (1962) Monographie des Menispermaceae Africaines. Mémoires in 8. Académie royale des sciences d’Outre-mer, classe des sciences naturelles et médicales, Nouvelle série
Spiff AI, Zabel V, Watson WH, Zemaitis MA, Ateya AM, Slatkin DJ, Knapp JE, Schiff PL Jr (1981) Constituents of West African medicinal plants. XXX. Tridictyophylline, a new morphinan alkaloid from Triclisia dictyophylla. J Nat Prod 44:160–165
Partridge SJ, Russell PF, Kirby GC, Warhurst DC, Phillipson JD, O’Neill MJ, Schiff PL (1989) In vitro antimalarial activity of Triclisia dictyophylla and some of its constituent alkaloids. J Pharm Pharmacol 41:92
Neuwinger HD (2000) African traditional medicine. A dictionary of plant use and applications. Medipharm Scientific Publishers, Stuttgart
Tackie AN, Dwuma-Badu D, Okarter T (1973) Trigilletine and tricordatine: two new bisbenzylisoquinoline alkaloids from Triclisia species. Phytochemistry 12:2509–2511
Tackie AN, Dwuma-Badu D, Okarter T, Knapp JE, Slatkin DJ, Schiff PL (1974) Constituents of West African medicinal plants. II. The isolation of alkaloids from selected Triclisia species. Lloydia 37:1–5
Dwuma-Badu D, Ayim JSK, Tackie AN, El Sohly MA, Knapp JE, Slatkin DJ, Schiff PL (1975) Trigilletimine: a new bisbenzylisoquinoline alkaloid from Triclisia species. Experientia 31:1251–1252
Ekong R, Partridge SJ, Anderson MM, Kirby GC, Warhurst DC, Russel PF, Phillipson JD (1991) Plasmodium falciparum: effects of phaeanthine, a naturally occurring bisbenzylisoquinoline alkaloid, on chloroquineresistant and choroquine-sensitive parasites in vitro, and its influence on chloroquine activity. Ann Trop Med Parasitol 85:205–213
Marshall SJ (1991) Antimalarial activities of some West African plants. Ph.D. thesis. In: Brighton Polytechnic and The School of Pharmacy, University of London, Brighton, UK
Azikiwe CC, Amazu LU, Unekwe PC, Nwosu PJ (2007) Anticoagulation and antithrombotic effects of Triclisia dictyophylla (moon seed). Niger J Nat Prod Med 11:29–31
Azikiwe CCA, Unekwe PC, Amazu LU, Srigernyaung L, Ezeani MC, Nwosu PJC, Ajugwo A (2009) Antitussive activity of Triclisia dictyophylla of the family Menispermecae. Asian Pac J Trop Med 2:33–40
Ajugwo AO, Ezimah AC (2013) In vivo studies of anticoagulation activity of Triclisia dictyophylla using albino wistar rats. Int J Pharm Sci Invent 2:37–40
Friesen JB, Pauli GF (2007) Rational development of solvent system families in counter-current chromatography. J Chromatogr A 1151:51–59
Skalicka-Wózniak K, Garrard I (2014) Counter-current chromatography for the separation of terpenoids: a comprehensive review with respect to the solvent systems employed. Phytochem Rev 13:547–572
Liu Y, Chen X, Liu J, Di D (2015) Three-phase solvent systems for the comprehensive separation of a wide variety of compounds from Dicranostigma leptopodum by high-speed counter-current chromatography. J Sep Sci 38:2038–2045
Friesen JB, McAlpine JB, Chen SN, Pauli GF (2015) Countercurrent separation of natural products: an update. J Nat Prod 78:1765–1796
Leitão GG, das Costa FN, Figueiredo FS (2012) Medicinal plants diversity and drugs, 1 edn. Science Publishers, Enfield
das Costa FN, Leitão GG (2010) Strategies of solvent system selection for the isolation of flavonoids by countercurrent chromatography. J Sep Sci 33:336–347
Yin L, Li Y, Lu B, Jia Y (2010) Trends in counter-current chromatography: applications to natural products purification. Sep Purif Rev 39:33–62
Ito Y (2013) pH-zone-refining counter-current chromatography: origin, mechanism, procedure and applications. J Chromatogr A 1271:71–85
Dong H, Zhang Y, Fang L, Duan W, Wang X, Huang L (2011) Combinative application of pH-zone-refining and conventional high-speed counter-current chromatography for preparative separation of alkaloids from Stephania kwangsiensis. J Chromatogr B 879:945–949
Zhenjia Z, Minglin W, Daijie W, Wenjuan D, Xiao W, Chengchao Z (2010) Preparative separation of alkaloids from Nelumbo nucifera leaves by conventional and pH-zone-refining counter-current chromatography. J Chromatogr B 878:1647–1651
Xu X, Sun CR, Dai XJ, Hu RL, Pan YJ, Yang ZF (2011) LC/MS Guided isolation of alkaloids from lotus leaves by pH-zone-refining counter-current chromatography. Molecules 16:2551–2560
Zhao J, Li P, Zheng Z, Pi Z, Xu L, Duan L, Ao W, Sun X, Liu Z, Liu J (2020) pH-Zone-refining counter-current chromatography for two new lipo-alkaloids separated from refined alkaline extraction of Kusnezoff monkshoodroot. J Sep Sci 13:1–12
Cruz RAS, Almeida H, Fernandes CP, Joseph-nathan P, Rocha L, Leitão GG (2016) A new tropane alkaloid from the leaves of Erythroxylum subsessile isolated by pH-zone refining counter-current chromatography. J Sep Sci 39:1273–1277
Zhang QL, Luan GX, Ma T, Hu N, Suo Y, Wang XY, Ma XF, Ding C (2015) Application of chromatography technology in the separation of active alkaloids from Hypecoum leptocarpum and their inhibitory effect on fatty acid synthase. J Sep Sci 38:4063–4070
Yu Q, Tong SQ, Yan JZ, Hong CQ, Zhai WF, Li YQ (2011) Preparative separation of quaternary ammonium alkaloids from Corydalis yanhusuo W. T. Wang by pH-zone-refining counter-current chromatography. J Sep Sci 34:278–285
Correa JE, Ríos CH, del Rosario CA, Romero LI, Ortega-Barría E, Coley PD, Kursar TA, Heller MV, Gerwick WH, Rios LC (2006) Minor alkaloids from Guatteria dumetorum with antileishmanial activity. Planta Med 72:270–272
Saidi N, Hadi AHA, Awang K, Mukhtar MR (2009) Aporphine alkaloids from bark of Cryptocarya ferrea. Indo J Chem 9:461–465
Tomita M, Furukawa H (1963) Studies on the alkaloids of Menispermaceous plants. CXCVI: alkaloids of Cocculus sarmentosus Diels. (4). The structure of water-soluble quaternary aporphine alkaloid “Cocsarmine.” Yakugaku Zasshi 83:190–194
Stévigny C, Jiwan JLH, Rozenberg R, de Hoffmann E, Quetin-Leclercq J (2004) Key fragmentation patterns of aporphine alkaloids by electrospray ionization with multistage mass spectrometry. Rapid Commun Mass Spectr 18:523–528
Acknowledgements
We are grateful to CNPq/TWAS post-doctoral fellowship (ELDK) and FAPERJ for financial support. GGL is indebted for the opportunity of presenting this work at CCC2016 Conference in Chicago, USA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have declared no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamto, E.L.D., Mendonça, S.C., Abouem A Zintchem, A. et al. Aporphine Alkaloids from Triclisia dictyophylla Diels by pH-Zone Refining Countercurrent Chromatography. Chromatographia 84, 13–20 (2021). https://doi.org/10.1007/s10337-020-03977-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03977-x