Abstract
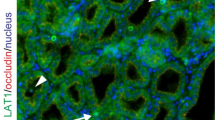
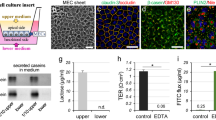
Mastitis causes a decrease in milk yield and abnormalities in milk components from dairy cows. Escherichia coli and the E. coli lipopolysaccharide (LPS) cell wall component directly downregulate milk production in bovine mammary epithelial cells (BMECs). However, the detailed mechanism by which this occurs in BMECs remains unclear. Various membrane proteins, such as immune sensors (Toll-like receptors, TLR), nutrient transporters (glucose transporter and aquaporin), and tight junction proteins (claudin and occludin) are involved in the onset of mastitis or milk production in BMECs. In this study, we investigated the influence of LPS on membrane proteins using an in vitro culture model. This mastitis model demonstrated a loss of glucose transporter-1 and aquaporin-3 at lateral membranes and a decrease in milk production in response to LPS treatment. LPS disrupted the tight junction barrier and caused compositional changes in localization of claudin-3 and claudin-4, although tight junctions were maintained to separate the apical membrane domains and the basolateral membrane domains. LPS did not significantly affect the expression level and subcellular localization of epidermal growth factor receptor in lactating BMECs with no detectable changes in MEK1/2-ERK1/2 signaling. In contrast, NFκB was concurrently activated with temporal translocation of TLR-4 in the apical membranes, whereas TLR-2 was not significantly influenced by LPS treatment. These findings indicate the importance of investigating the subcellular localization of membrane proteins to understand the molecular mechanism of LPS in milk production in mastitis.







Similar content being viewed by others
Abbreviations
- AQP:
-
Aquaporin
- BMEC:
-
Bovine mammary epithelial cell
- BSA:
-
Bovine serum albumin
- EGFR:
-
Epidermal growth factor receptor
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GLUT1:
-
Glucose transporter-1
- LPS:
-
Lipopolysaccharide
- PBS-T:
-
PBS containing 0.05% Tween 20
- STAT:
-
Signal transducer and activator of transcription
- SDS:
-
Sodium dodecyl sulfate
- TJ:
-
Tight junction
- TLR:
-
Toll-like receptor
References
Abate W, Alghaithy AA, Parton J, Jones KP, Jackson SK (2010) Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J Lipid Res 51:334–344
Adams KM, Lucas J, Kapur RP, Stevens AM (2007) LPS induces translocation of TLR4 in amniotic epithelium. Placenta 28:477–481
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406:782–787
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680
Anderson SM, Rudolph MC, McManaman JL, Neville MC (2007) Key stages in mammary gland development. Secretory activation in the mammary gland: It's not just about milk protein synthesis! Breast Cancer Res 9:204
Baumgartner HK, Rudolph MC, Ramanathan P, Burns V, Webb P, Bitler BG, Stein T, Kobayashi K, Neville MC (2017) Developmental expression of claudins in the mammary gland. J Mammary Gland Biol Neoplasia 22:141–157
Bouley R, Hawthorn G, Russo LM, Lin HY, Ausiello DA, Brown D (2006) Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in “endocytosis-resistant” membrane domains after vasopressin treatment. Biol Cell 98:215–232
Camps M, Vilaro S, Testar X, Palacin M, Zorzano A (1994) High and polarized expression of GLUT1 glucose transporters in epithelial cells from mammary gland: Acute down-regulation of GLUT1 carriers by weaning. Endocrinology 134:924–934
Cereijido M, Valdes J, Shoshani L, Contreras RG (1998) Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol 60:161–177
Derakhshani H, Fehr KB, Sepehri S, Francoz D, De Buck J, Barkema HW, Plaizier JC, Khafipour E (2018) Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J Dairy Sci 101:10605–10625
Dill R, Walker AM (2017) Role of prolactin in promotion of immune cell migration into the mammary gland. J Mammary Gland Biol Neoplasia 22:13–26
Gross JJ, van Dorland HA, Wellnitz O, Bruckmaier RM (2015) Glucose transport and milk secretion during manipulated plasma insulin and glucose concentrations and during LPS-induced mastitis in dairy cows. J Anim Physiol Anim Nutr (Berl) 99:747–756
Halasa T, Huijps K, Osteras O, Hogeveen H (2007) Economic effects of bovine mastitis and mastitis management: A review. Vet Q 29:18–31
Hughes K, Watson CJ (2018) The mammary microenvironment in mastitis in humans, dairy ruminants, rabbits and rodents: A one health focus. J Mammary Gland Biol Neoplasia 23:27–41
Ingman WV, Glynn DJ, Hutchinson MR (2014) Inflammatory mediators in mastitis and lactation insufficiency. J Mammary Gland Biol Neoplasia 19:161–167
Jiang A, Zhang Y, Zhang X, Wu D, Liu Z, Li S, Liu X, Han Z, Wang C, Wang J, Wei Z, Guo C, Yang Z (2020) Morin alleviates LPS-induced mastitis by inhibiting the PI3K/AKT, MAPK, NF-kappaB and NLRP3 signaling pathway and protecting the integrity of blood-milk barrier. Int Immunopharmacol 78:105972
Johnzon CF, Dahlberg J, Gustafson AM, Waern I, Moazzami AA, Ostensson K, Pejler G (2018) The effect of lipopolysaccharide-induced experimental bovine mastitis on clinical parameters, inflammatory markers, and the metabolome: A kinetic approach. Front Immunol 9:1487
Kaihoko Y, Tsugami Y, Suzuki N, Suzuki T, Nishimura T, Kobayashi K (2020) Distinct expression patterns of aquaporin 3 and 5 in ductal and alveolar epithelial cells in mouse mammary glands before and after parturition. Cell Tissue Res 380:513–526
Kobayashi K, Miwa H, Yasui M (2010) Inflammatory mediators weaken the amniotic membrane barrier through disruption of tight junctions. J Physiol 588:4859–4869
Kobayashi K, Oyama S, Numata A, Rahman MM, Kumura H (2013) Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PLoS ONE 8:e62187
Kobayashi K, Oyama S, Uejyo T, Kuki C, Rahman MM, Kumura H (2013) Underlying mechanisms involved in the decrease of milk secretion during Escherichia coli endotoxin induced mastitis in lactating mice. Vet Res 44:119
Ling B, Alcorn J (2010) LPS-induced inflammation downregulates mammary gland glucose, fatty acid, and L-carnitine transporter expression at different lactation stages. Res Vet Sci 89:200–202
Ludewig T (1996) Light and electron microscopic investigations of the blood-milk barrier in lactating cow udders. Anat Histol Embryol 25:121–126
Matsunaga K, Tsugami Y, Kumai A, Suzuki T, Nishimura T, Kobayashi K (2018) IL-1beta directly inhibits milk lipid production in lactating mammary epithelial cells concurrently with enlargement of cytoplasmic lipid droplets. Exp Cell Res 370:365–372
Miao J, Fa Y, Gu B, Zhu W, Zou S (2012) Taurine attenuates lipopolysaccharide-induced dysfunction in mouse mammary epithelial cells. Cytokine 59:35–40
Mobasheri A, Kendall BH, Maxwell JE, Sawran AV, German AJ, Marples D, Luck MR, Royal MD (2011) Cellular localization of aquaporins along the secretory pathway of the lactating bovine mammary gland: An immunohistochemical study. Acta Histochem 113:137–149
Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC (2001) Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem 276:35571–35580
Pauloin A, Chanat E (2012) Prolactin and epidermal growth factor stimulate adipophilin synthesis in HC11 mouse mammary epithelial cells via the PI3-kinase/Akt/mTOR pathway. Biochim Biophys Acta 1823:987–996
Riskin A, Nannegari VH, Mond Y (2008) Acute effectors of GLUT1 glucose transporter subcellular targeting in CIT3 mouse mammary epithelial cells. Pediatr Res 63:56–61
Rodriguez RA, Liang H, Chen LY, Plascencia-Villa G, Perry G (2019) Single-channel permeability and glycerol affinity of human aquaglyceroporin AQP3. Biochim Biophys Acta Biomembr 1861:768–775
Seegers H, Fourichon C, Beaudeau F (2003) Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res 34:475–491
Shin K, Fogg VC, Margolis B (2006) Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22:207–235
Stewart TA, Hughes K, Hume DA, Davis FM (2019) Developmental stage-specific distribution of macrophages in mouse mammary gland. Front Cell Dev Biol 7:250
Truchet S, Ollivier-Bousquet M (2009) Mammary gland secretion: Hormonal coordination of endocytosis and exocytosis. Animal 3:1733–1742
Tsugami Y, Suzuki N, Kawahara M, Suzuki T, Nishimura T, Kobayashi K (2020) Establishment of an in vitro culture model to study milk production and the blood-milk barrier with bovine mammary epithelial cells. Anim Sci J 91:e13355
Tsugami Y, Suzuki N, Suzuki T, Nishimura T, Kobayashi K (2020) Regulatory effects of soy isoflavones and their metabolites in milk production via different ways in mice. J Agric Food Chem 68:5847–5853
Wang D, Cai J, Zhao F, Liu J (2019) Low-quality rice straw forage increases the permeability of mammary epithelial tight junctions in lactating dairy cows. J Sci Food Agric 99:2037–2041
Wang JH, Du JY, Wu YY, Chen MC, Huang CH, Shen HJ, Lee CF, Lin TH, Lee YJ (2014) Suppression of prolactin signaling by pyrrolidine dithiocarbamate is alleviated by N-acetylcysteine in mammary epithelial cells. Eur J Pharmacol 738:301–309
Watson CJ, Burdon TG (1996) Prolactin signal transduction mechanisms in the mammary gland: The role of the Jak/Stat pathway. Rev Reprod 1:1–5
Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G, Shen CH, Wen J, Asara J, McGraw TE, Kahn BB, Cantley LC (2013) AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 49:1167–1175
Yasui H, Kubota M, Iguchi K, Usui S, Kiho T, Hirano K (2008) Membrane trafficking of aquaporin 3 induced by epinephrine. Biochem Biophys Res Commun 373:613–617
Zhao FQ, Keating AF (2007) Expression and regulation of glucose transporters in the bovine mammary gland. J Dairy Sci 90(Suppl 1):E76-86
Acknowledgments
We are grateful to Kota Matsunaga, Naoki Omatsu, and Futa Shinagawa, Laboratory of Dairy Food Science, Research, Faculty of Agriculture, Hokkaido University, for their helpful research advice.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS Kakenhi Grant Number 18H0232009 and JP19J10189).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved by the Animal Resource Committee of Hokkaido University (permission number: 15–0085).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsugami, Y., Wakasa, H., Kawahara, M. et al. Adverse effects of LPS on membrane proteins in lactating bovine mammary epithelial cells. Cell Tissue Res 384, 435–448 (2021). https://doi.org/10.1007/s00441-020-03344-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03344-0