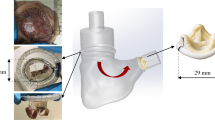
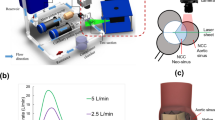
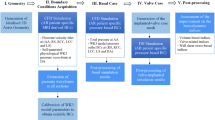
Abstract
Purpose
Discrete subaortic stenosis (DSS) is a left-ventricular outflow tract (LVOT) obstruction caused by a membranous lesion. DSS is associated with steep aortoseptal angles (AoSAs) and is a risk factor for aortic regurgitation (AR). However, the etiology of AR secondary to DSS remains unknown. This study aimed at quantifying computationally the impact of AoSA steepening and DSS on aortic valve (AV) hemodynamics and AR.
Methods
An LV geometry reconstructed from cine-MRI data was connected to an AV geometry to generate a unified 2D LV-AV model. Six geometrical variants were considered: unobstructed (CTRL) and DSS-obstructed LVOT (DSS), each reflecting three AoSA variations (110°, 120°, 130°). Fluid-structure interaction simulations were run to compute LVOT flow, AV leaflet dynamics, and regurgitant fraction (RF).
Results
AoSA steepening and DSS generated vortex dynamics alterations and stenotic flow conditions. While the CTRL-110° model generated the highest degree of leaflet opening asymmetry, DSS preferentially altered superior leaflet kinematics, and caused leaflet-dependent alterations in systolic fluttering. LVOT steepening and DSS subjected the leaflets to increasing WSS overloads (up to 94% increase in temporal shear magnitude), while DSS also increased WSS bidirectionality on the inferior leaflet belly (+ 0.30-point in oscillatory shear index). Although AoSA steepening and DSS increased diastolic transvalvular backflow, regurgitant fractions (RF < 7%) remained below the threshold defining clinical mild AR.
Conclusions
The mechanical interactions between AV leaflets and LVOT steepening/DSS hemodynamic derangements do not cause AR. However, the leaflet WSS abnormalities predicted in those anatomies provide new support to a mechanobiological etiology of AR secondary to DSS.





Similar content being viewed by others
Abbreviations
- ALE:
-
Arbitrary Lagrangian–Eulerian
- AoSA:
-
Aortoseptal angle
- AR:
-
Aortic regurgitation
- AV:
-
Aortic valve
- CCW:
-
Counterclockwise
- CTRL:
-
Control group
- CW:
-
Clockwise
- DSS:
-
Discrete subaortic stenosis
- EOA:
-
Effective orifice area
- FSI:
-
Fluid-structure interaction
- LV:
-
Left ventricle
- LVOT:
-
Left ventricular outflow tract
- OSI:
-
Oscillatory shear index
- RF:
-
Regurgitant fraction
- RMS:
-
Root mean square
- TSM:
-
Temporal shear magnitude
- WSS:
-
Wall shear stress
References
Aboulhosn, J., and J. S. Child. Left ventricular outflow obstruction: Subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, and coarctation of the aorta. Circulation 114(22):2412–2422, 2006. https://doi.org/10.1161/CIRCULATIONAHA.105.592089.
Amindari, A., L. Saltik, K. Kirkkopru, M. Yacoub, and H. C. Yalcin. Assessment of calcified aortic valve leaflet deformations and blood flow dynamics using fluid-structure interaction modeling. Inf. Med. Unlocked 9:191–199, 2017. https://doi.org/10.1016/j.imu.2017.09.001.
Annerel, S., J. Degroote, T. Claessens, P. Segers, P. Verdonck, and J. Vierendeels. The upstream boundary condition influences the leaflet opening dynamics in the numerical FSI simulation of an aortic BMHV. Int. J. Numer. Methods Biomed. Eng. 28(6–7):745–760, 2012. https://doi.org/10.1002/cnm.2470.
Atkins, S. K., A. McNally, and P. Sucosky. Mechanobiology in cardiovascular disease management: potential strategies and current needs. Front. Bioeng. Biotechnol. 4:79, 2016. https://doi.org/10.3389/fbioe.2016.00079.
Balachandran, K., P. Sucosky, and A. P. Yoganathan. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int. J. Inflamm. 2011:263870, 2011. https://doi.org/10.4061/2011/263870.
Barboza, L. A., F. M. Garcia, J. Barnoya, J. R. Leon-Wyss, and A. R. Castañeda. Subaortic membrane and aorto-septal angle: an echocardiographic assessment and surgical outcome. World J. Pediatr. Congenital Heart Surg. 4(3):253–261, 2013. https://doi.org/10.1177/2150135113485760.
Bavo, A. M., A. M. Pouch, J. Degroote, J. Vierendeels, J. H. Gorman, R. C. Gorman, and P. Segers. Patient-specific CFD simulation of intraventricular haemodynamics based on 3D ultrasound imaging. BioMed. Eng. Online 15(1):107, 2016. https://doi.org/10.1186/s12938-016-0231-9.
Bruening, J., F. Hellmeier, P. Yevtushenko, M. Kelm, S. Nordmeyer, S. H. Sündermann, T. Kuehne, and L. Goubergrits. Impact of patient-specific LVOT inflow profiles on aortic valve prosthesis and ascending aorta hemodynamics. J. Comput. Sci. 24:91–100, 2018. https://doi.org/10.1016/j.jocs.2017.11.005.
Butany, J., P. Vaideeswar, and T. E. David. Discrete subaortic membranes in adults—a clinicopathological analysis. Cardiovasc. Pathol. 18(4):236–242, 2009. https://doi.org/10.1016/j.carpath.2008.06.013.
Campbell, M. J., and T. D. V. Swinscow. Statistics at Square One (11th ed.). London: BMJ Books, 2009.
Cao, K., M. Bukač, and P. Sucosky. Three-dimensional macro-scale assessment of regional and temporal wall shear stress characteristics on aortic valve leaflets. Comput. Methods Biomech. Biomed. Eng. 19(6):603–613, 2016. https://doi.org/10.1080/10255842.2015.1052419.
Cao, K., and P. Sucosky. Computational comparison of regional stress and deformation characteristics in tricuspid and bicuspid aortic valve leaflets. Int. J. Numer. Methods Biomed. Eng. 33(3):e02798, 2017. https://doi.org/10.1002/cnm.2798.
Cape, E. G., M. D. Vanauker, G. Sigfússon, T. A. Tacy, and P. J. del Nido. Potential role of mechanical stress in the etiology of pediatric heart disease: septal shear stress in subaortic stenosis. J. Am. Coll. Cardiol. 30(1):247–254, 1997.
Carmody, C. J., G. Burriesci, I. C. Howard, and E. A. Patterson. An approach to the simulation of fluid-structure interaction in the aortic valve. J. Biomech. 39(1):158–169, 2006. https://doi.org/10.1016/j.jbiomech.2004.10.038.
Chandra, S., N. M. Rajamannan, and P. Sucosky. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech. Model. Mechanobiol. 11(7):1085–1096, 2012. https://doi.org/10.1007/s10237-012-0375-x.
Chandra, S., S. S. Raut, A. Jana, R. W. Biederman, M. Doyle, S. C. Muluk, and E. A. Finol. Fluid-structure interaction modeling of abdominal aortic aneurysms: the impact of patient-specific inflow conditions and fluid/solid coupling. J. Biomech. Eng. 135(8):081001, 2013. https://doi.org/10.1115/1.4024275.
Chaothawee, L. Diagnostic approach to assessment of valvular heart disease using magnetic resonance imaging. part II: a practical approach for native and prosthetic heart valve stenosis. Heart Asia 4(1):171–175, 2012. https://doi.org/10.1136/heartasia-2012-010124.
Chnafa, C., S. Mendez, and F. Nicoud. Image-based large-eddy simulation in a realistic left heart. Comput. Fluids 94:173–187, 2014. https://doi.org/10.1016/j.compfluid.2014.01.030.
Dasi, L. P., L. Ge, H. A. Simon, F. Sotiropoulos, and A. P. Yoganathan. Vorticity dynamics of a bileaflet mechanical heart valve in an axisymmetric aorta. Phys. Fluids 19(6):067105, 2007. https://doi.org/10.1063/1.2743261.
de Vries, A. G., J. Hess, M. Witsenburg, I. M. Frohn-Mulder, J. J. Bogers, and E. Bos. Management of fixed subaortic stenosis: a retrospective study of 57 cases. J. Am. Coll. Cardiol. 19(5):1013–1017, 1992. https://doi.org/10.1016/0735-1097(92)90286-v.
Donea, J., S. Guiliani, and J. P. Halleux. An arbitrary Lagrangian–Eulerian finite-element method for transient dynamic fluid structure interactions. Comput. Methods Appl. Mech. Eng. 33(1–3):689–723, 1982. https://doi.org/10.1016/0045-7825(82)90128-1.
Doost, S. N., L. Zhong, B. Su, and Y. S. Morsi. Two-dimensional intraventricular flow pattern visualization using the image-based computational fluid dynamics. Comput. Methods Biomech. Biomed. Eng. 20(5):492–507, 2017. https://doi.org/10.1080/10255842.2016.1250891.
Erentug, V., N. Bozbuga, K. Kirali, D. Goksedef, E. Akinci, Ö. Isik, and C. Yakut. Surgical treatment of subaortic obstruction in adolescent and adults: long-term follow-up. J. Card. Surg. 20(1):16–21, 2005. https://doi.org/10.1111/j.0886-0440.2005.200336.x.
Ezon, D. S. Fixed subaortic stenosis: a clinical dilemma for clinicians and patients. Congenital Heart Dis. 8(5):450–456, 2013. https://doi.org/10.1111/chd.12127.
Feigl, A., D. Feigl, R. V. Lucas, and J. E. Edwards. Involvement of the aortic valve cusps in discrete subaortic stenosis. Pediatr. Cardiol. 5(3):185–189, 1984.
Foker, J. E. Outcomes and questions about discrete subaortic stenosis. Circulation 127(14):1447–1450, 2013. https://doi.org/10.1161/CIRCULATIONAHA.113.001619.
Guivier, C., V. Deplano, and P. Pibarot. New insights into the assessment of the prosthetic valve performance in the presence of subaortic stenosis through a fluid-structure interaction model. J. Biomech. 40(10):2283–2290, 2007. https://doi.org/10.1016/j.jbiomech.2006.10.010.
Heiberg, E., J. Sjögren, M. Ugander, M. Carlsson, H. Engblom, and H. Arheden. Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med. Imaging. 2010. https://doi.org/10.1186/1471-2342-10-1.
Hoehn, D., L. Sun, and P. Sucosky. Role of pathologic shear stress alterations in aortic valve endothelial activation. Cardiovasc. Eng. Technol. 1(2):165–178, 2010. https://doi.org/10.1007/s13239-010-0015-5.
Hoeijmakers, M. J. M. M., D. A. Silva Soto, I. Waechter-Stehle, M. Kasztelnik, J. Weese, D. R. Hose, and F. N. van de Vosse. Estimation of valvular resistance of segmented aortic valves using computational fluid dynamics. J. Biomech. 94:49–58, 2019. https://doi.org/10.1016/j.jbiomech.2019.07.010.
Hung, T.-K., S. S. Khalafvand, and E. Y. K. Naaag. Fluid dynamic characteristics of systolic blood flow of the left ventricle. J. Mech. Med. Biol. 2015. https://doi.org/10.1142/s0219519415500475.
Imanparast, A., N. Fatouraee, and F. Sharif. The impact of valve simplifications on left ventricular hemodynamics in a three dimensional simulation based on in vivo MRI data. J. Biomech. 49(9):1482–1489, 2016. https://doi.org/10.1016/j.jbiomech.2016.03.021.
Keshavarz-Motamed, Z., S. Khodaei, F. Rikhtegar Nezami, J. M. Amrute, S. J. Lee, J. Brown, E. Ben-Assa, T. Garcia Camarero, J. Ruano Calvo, S. Sellers, P. Blanke, J. Leipsic, J. M. de la Torre Hernandez, and E. R. Edelman. Mixed valvular disease following transcatheter aortic valve replacement: quantification and systematic differentiation using clinical measurements and image-based patient-specific in silico modeling. JAHA. 2020. https://doi.org/10.1161/jaha.119.015063.
Khalafvand, S. S., J. D. Voorneveld, A. Muralidharan, F. J. H. Gijsen, J. G. Bosch, T. van Walsum, A. Haak, N. de Jong, and S. Kenjeres. Assessment of human left ventricle flow using statistical shape modelling and computational fluid dynamics. J. Biomech. 74:116–125, 2018. https://doi.org/10.1016/j.jbiomech.2018.04.030.
Kleinert, S., and T. Geva. Echocardiographic morphometry and geometry of the left ventricular outflow tract in fixed subaortic stenosis. J. Am. Coll. Cardiol. 22(5):1501–1508, 1993. https://doi.org/10.1016/0735-1097(93)90563-G.
Kratochvíl, F., T. Paleček, T. Grus, and P. Kuchynka. Fixed subaortic stenosis. Cor. Vasa. 59(5):e436–e440, 2017. https://doi.org/10.1016/j.crvasa.2016.10.001.
Krueger, S. K., J. W. French, A. D. Forker, C. C. Caudill, and R. L. Popp. Echocardiography in discrete subaortic stenosis. Circulation 59(3):506–513, 1979. https://doi.org/10.1161/01.CIR.59.3.506.
Le, T. B., and F. Sotiropoulos. Fluid-structure interaction of an aortic heart valve prosthesis driven by an animated anatomic left ventricle. J. Comput. Phys. 244:41–62, 2013. https://doi.org/10.1016/j.jcp.2012.08.036.
Liu, J., K. Cornelius, M. Graham, T. Leonard, A. Tipton, A. Yorde, and P. Sucosky. Design and computational validation of a novel bioreactor for conditioning vascular tissue to time-varying multidirectional fluid shear stress. Cardiovasc. Eng. Technol. 10(3):531–542, 2019. https://doi.org/10.1007/s13239-019-00426-1.
Mao, W., A. Caballero, R. McKay, C. Primiano, and W. Sun. Fully-coupled fluid-structure interaction simulation of the aortic and mitral valves in a realistic 3D left ventricle model. PLoS ONE 2017. https://doi.org/10.1371/journal.pone.0184729.
Marasini, M., L. Zannini, G. P. Ussia, R. Pinto, R. Moretti, F. Lerzo, and G. Pongiglione. Discrete subaortic stenosis: Incidence, morphology and surgical impact of associated subaortic anomalies. Ann. Thorac. Surg. 75(6):1763–1768, 2003. https://doi.org/10.1016/S0003-4975(02)05027-0.
Massé, D. D., J. A. Shar, K. N. Brown, S. G. Keswani, K. J. Grande-Allen, and P. Sucosky. Discrete subaortic stenosis: perspective roadmap to a complex disease. Front. Cardiovasc. Med. 2018. https://doi.org/10.3389/fcvm.2018.00122.
Neto, F. L., L. C. Marques, and V. D. Aiello. Myxomatous degeneration of the mitral valve. Autops Case Rep. 2018. https://doi.org/10.4322/acr.2018.058.
Nishimura, R. A. doppler echocardiography: theory, instrumentation, technique, and application. Mayo Clin. Proc. 60:23, 1985.
O’Leary, C. A., and I. Wilkie. Cardiac valvular and vascular disease in Bull Terriers. Vet. Pathol. 46(6):1149–1155, 2009. https://doi.org/10.1354/vp.06-VP-0230-O-FL.
Oliver, J. M., A. González, P. Gallego, A. Sánchez-Recalde, F. Benito, and J. M. Mesa. Discrete subaortic stenosis in adults: Increased prevalence and slow rate of progression of the obstruction and aortic regurgitation. J. Am. Coll. Cardiol. 38(3):835–842, 2001. https://doi.org/10.1016/S0735-1097(01)01464-4.
Opotowsky, A. R., S. S. Pickard, and T. Geva. Imaging adult patients with discrete subvalvar aortic stenosis. Curr. Opin. Cardiol. 32(5):513–520, 2017. https://doi.org/10.1097/HCO.0000000000000425.
Ozsin, K. K., F. Toktas, U. S. Sanri, and S. Yavuz. Discrete subaortic stenosis in an adult patient. Eur. Res. J. 2(1):66–70, 2016. https://doi.org/10.18621/eurj.2016.2.1.66.
Parry, A. J., J. P. Kovalchin, K. Suda, D. B. McElhinney, J. Wudel, N. H. Silverman, V. M. Reddy, and F. L. Hanley. Resection of subaortic stenosis; can a more aggressive approach be justified? Eur. J. Cardiothorac. Surg. 15(5):631–638, 1999. https://doi.org/10.1016/S1010-7940(99)00060-3.
Pickard, S. S., A. Geva, K. Gauvreau, P. J. Del Nido, and T. Geva. Long-term outcomes and risk factors for aortic regurgitation after discrete subvalvular aortic stenosis resection in children. Heart 101(19):1547–1553, 2015. https://doi.org/10.1136/heartjnl-2015-307460.
Rathan, S., C. J. Ankeny, S. Arjunon, Z. Ferdous, S. Kumar, J. Fernandez Esmerats, J. M. Heath, R. M. Nerem, A. P. Yoganathan, and H. Jo. Identification of side- and shear-dependent microRNAs regulating porcine aortic valve pathogenesis. Sci Rep 2016. https://doi.org/10.1038/srep25397.
Samian, R., and M. Saidi. Investigation of left heart flow using a numerical correlation to model heart wall motion. J. Biomech. 93:77–85, 2019. https://doi.org/10.1016/j.jbiomech.2019.06.008.
Schenkel, T., M. Malve, M. Reik, M. Markl, B. Jung, and H. Oertel. MRI-Based CFD analysis of flow in a human left ventricle: methodology and application to a healthy heart. Ann. Biomed. Eng. 37(3):503–515, 2009. https://doi.org/10.1007/s10439-008-9627-4.
Schneider, P. J., and J. D. Deck. Tissue and cell renewal in the natural aortic valve of rats: an autoradiographic study. Cardiovasc. Res. 15(4):181–189, 1981. https://doi.org/10.1093/cvr/15.4.181.
Shar, J. A., K. N. Brown, S. G. Keswani, J. Grande-Allen, and P. Sucosky. Impact of aortoseptal angle abnormalities and discrete subaortic stenosis on left-ventricular outflow tract hemodynamics: preliminary computational assessment. Front. Bioeng. Biotechnol. 8:114, 2020. https://doi.org/10.3389/fbioe.2020.00114.
Sigfússon, G., T. A. Tacy, M. D. Vanauker, and E. G. Cape. Abnormalities of the left ventricular outflow tract associated with discrete subaortic stenosis in children: An echocardiographic study. J. Am. Coll. Cardiol. 30(1):255–259, 1997. https://doi.org/10.1016/S0735-1097(97)00151-4.
Stassano, P., L. Di Tommaso, A. Contaldo, M. Monaco, M. Mottola, A. Musumeci, G. Coronella, and N. Spampinato. Discrete subaortic stenosis: long-term prognosis on the progression of the obstruction and of the aortic insufficiency. Thorac. Cardiovasc. Surg. 53(1):23–27, 2005. https://doi.org/10.1055/s-2004-830388.
Stupak, E., R. Kačianauskas, A. Kačeniauskas, V. Starikovičius, A. Maknickas, R. Pacevič, M. Stašku, G. Davidavičius, and A. Aidietis. The geometric model-based patient-specific simulations of turbulent aortic valve flows. Archiv. Mech. 69(4–5):29, 2017.
Su, B., R. S. Tan, J. L. Tan, K. W. Q. Guo, J. M. Zhang, S. Leng, X. Zhao, J. C. Allen, and L. Zhong. Cardiac MRI based numerical modeling of left ventricular fluid dynamics with mitral valve incorporated. J. Biomech. 49(7):1199–1205, 2016. https://doi.org/10.1016/j.jbiomech.2016.03.008.
Su, B., L. Zhong, X. K. Wang, J. M. Zhang, R. S. Tan, J. C. Allen, S. K. Tan, S. Kim, and H. L. Leo. Numerical simulation of patient-specific left ventricular model with both mitral and aortic valves by FSI approach. Comput. Methods Programs Biomed. 113(2):474–482, 2014. https://doi.org/10.1016/j.cmpb.2013.11.009.
Sucosky, P., K. Balachandran, A. Elhammali, H. Jo, and A. P. Yoganathan. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29(2):254–260, 2009. https://doi.org/10.1161/ATVBAHA.108.176347.
Sucosky, P., M. Padala, A. Elhammali, K. Balachandran, H. Jo, and A. P. Yoganathan. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J. Biomech. Eng. 130(3):35001–35008, 2008. https://doi.org/10.1115/1.2907753.
Sucosky, P., J. A. Shar, and J. Barrientos. Cardiovascular mechanics and disease. In: Mechanobiology, edited by G. L. Niebur. New York: Elsevier, 2020.
Sun, L., S. Chandra, and P. Sucosky. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS ONE 7(10):e48843, 2012. https://doi.org/10.1371/journal.pone.0048843.
Sun, L., N. M. Rajamannan, and P. Sucosky. Design and validation of a novel bioreactor to subject aortic valve leaflets to side-specific shear stress. Ann. Biomed. Eng. 39(8):2174–2185, 2011. https://doi.org/10.1007/s10439-011-0305-6.
Sun, L., N. M. Rajamannan, and P. Sucosky. Defining the role of fluid shear stress in the expression of early signaling markers for calcific aortic valve disease. PLoS ONE 8(12):e84433, 2013. https://doi.org/10.1371/journal.pone.0084433.
Sun, L., and P. Sucosky. Bone morphogenetic protein-4 and transforming growth factor-beta1 mechanisms in acute valvular response to supra-physiologic hemodynamic stresses. World J. Cardiol. 7(6):331–343, 2015. https://doi.org/10.4330/wjc.v7.i6.331.
Thubrikar, M. The aortic valve. Boca Raton: CRC Press, 1990.
Van Der Linde, D., J. W. Roos-Hesselink, D. Rizopoulos, H. J. Heuvelman, W. Budts, A. P. J. Van Dijk, M. Witsenburg, S. C. Yap, A. Oxenius, C. K. Silversides, E. N. Oechslin, A. J. J. C. Bogers, and J. J. M. Takkenberg. Surgical outcome of discrete subaortic stenosis in adults a multicenter study. Circulation 127(11):1184–1191, 2013. https://doi.org/10.1161/CIRCULATIONAHA.112.000883.
Weinberg, E. J., and M. R. Kaazempur Mofrad. Transient, three-dimensional, multiscale simulations of the human aortic valve. Cardiovasc. Eng. 7(4):140–155, 2007. https://doi.org/10.1007/s10558-007-9038-4.
Weston, M. W., D. V. LaBorde, and A. P. Yoganathan. Estimation of the shear stress on the surface of an aortic valve leaflet. Ann. Biomed. Eng. 27(4):572–579, 1999. https://doi.org/10.1114/1.199.
Willems, I. E., M. G. Havenith, J. F. Smits, and M. J. Daemen. Structural alterations in heart valves during left ventricular pressure overload in the rat. Lab. Invest. 71(1):127–133, 1994.
Wright, G. B., J. F. Keane, A. S. Nadas, W. F. Bernhard, and A. R. Castaneda. Fixed subaortic stenosis in the young: medical and surgical course in 83 patients. Am. J. Cardiol. 52(7):830–835, 1983. https://doi.org/10.1016/0002-9149(83)90423-x.
Yap, S.-C., J. W. Roos-Hesselink, A. J. J. C. Bogers, and F. J. Meijboom. Steepened aortoseptal angle may be a risk factor for discrete subaortic stenosis in adults. Int. J. Cardiol. 126(1):138–139, 2008. https://doi.org/10.1016/j.ijcard.2007.01.078.
Yap, C. H., N. Saikrishnan, G. Tamilselvan, and A. P. Yoganathan. Experimental measurement of dynamic fluid shear stress on the ventricular surface of the aortic valve leaflet. Biomech. Model. Mechanobiol. 11(1–2):171–182, 2011. https://doi.org/10.1007/s10237-011-0301-7.
Zoghbi, W. A., D. Adams, R. O. Bonow, M. Enriquez-Sarano, E. Foster, P. A. Grayburn, R. T. Hahn, Y. Han, J. Hung, R. M. Lang, S. H. Little, D. J. Shah, S. Shernan, P. Thavendiranathan, J. D. Thomas, and N. J. Weissman. Recommendations for noninvasive evaluation of native valvular regurgitation. J. Am. Soc. Echocardiogr. 30(4):303–371, 2017. https://doi.org/10.1016/j.echo.2017.01.007.
Acknowledgments
The authors would like to thank Dr. Matt Sherwood and Mr. Aaron Madaris (Wright State University) for their assistance in acquiring the MRI data.
Funding
This work was supported in part by the National Institutes of Health (NIH) Grant R01HL140305, and the College of Engineering and Computer Science at Wright State University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Information 2 (MP4 6835 kb)
Rights and permissions
About this article
Cite this article
Shar, J.A., Keswani, S.G., Grande-Allen, K.J. et al. Computational Assessment of Valvular Dysfunction in Discrete Subaortic Stenosis: A Parametric Study. Cardiovasc Eng Tech 12, 559–575 (2021). https://doi.org/10.1007/s13239-020-00513-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00513-8