Initial In Vivo Evaluation of a Novel Amikacin-Deoxycholate Hydrophobic Salt Delivers New Insights on Amikacin Partition in Blood and Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Amk-DCA Hydrophobic Salt
2.3. Animals
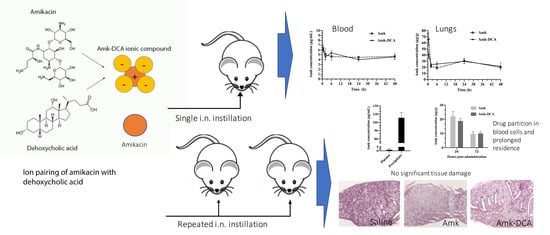
2.4. Pharmacokinetic Study
2.5. Repeated Administration Regimen
2.6. Inflammatory Cytokines and Histology
2.7. Sample Preparation and Analysis
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Spencer, S.; Felix, L.M.; Milan, S.J.; Normansell, R.; Goeminne, P.C.; Chalmers, J.D.; Donovan, T. Oral versus inhaled antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018, 3, CD012579. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Schoubben, A.; Ricci, M. The long and winding road to inhaled TB therapy: Not only the bug’s fault. Drug Dev. Ind. Pharm. 2017, 43, 347–363. [Google Scholar] [CrossRef]
- Fattorini, L.; Piccaro, G.; Mustazzolu, A.; Giannoni, F. Targeting dormant bacilli to fight tuberculosis. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013072. [Google Scholar] [CrossRef]
- Piccaro, G.; Poce, G.; Biava, M.; Giannoni, F.; Fattorini, L. Activity of lipophilic and hydrophilic drugs against dormant and replicating Mycobacterium tuberculosis. J. Antibiot. (Tokyo) 2015, 68, 711–714. [Google Scholar] [CrossRef] [Green Version]
- Iacobino, A.; Piccaro, G.; Giannoni, F.; Mustazzolu, A.; Fattorini, L. Fighting tuberculosis by drugs targeting nonreplicating Mycobacterium tuberculosis bacilli. Int. J. Mycobacteriol. 2017, 6, 213–221. [Google Scholar]
- Cazzola, M.; D’Amato, G.; Matera, M.G. Intrapulmonary penetration of antimicrobials and implications in the treatment of lower respiratory tract infections. In Antibiotics and the Lung; European Respiratory Society: Lausanne, Switzerland, 2004; pp. 13–44. [Google Scholar]
- Kempker, R.R.; Heinrichs, M.T.; Nikolaishvili, K.; Sabulua, I.; Bablishvili, N.; Gogishvili, S.; Avaliani, Z.; Tukvadze, N.; Little, B.; Bernheim, A.; et al. Lung tissue concentrations of pyrazinamide among patients with drug-resistant pulmonary tuberculosis. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoubben, A.; Blasi, P.; Marenzoni, M.L.; Barberini, L.; Giovagnoli, S.; Cirotto, C.; Ricci, M. Capreomycin supergenerics for pulmonary tuberculosis treatment: Preparation, in vitro, and in vivo characterization. Eur. J. Pharm. Biopharm. 2013, 83, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, S.; Marenzoni, M.L.; Nocchetti, M.; Santi, C.; Blasi, P.; Schoubben, A.; Ricci, M. Synthesis, characterization and in vitro extracellular and intracellular activity against Mycobacterium tuberculosis infection of new second-line antitubercular drug-palladium complexes. J. Pharm. Pharmacol. 2014, 66, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, F.; Giovagnoli, S.; Schoubben, A.; Blasi, P.; Rossi, C.; Ricci, M. Development of a spray-drying method for the formulation of respirable microparticles containing ofloxacin-palladium complex. Int. J. Pharm. 2013, 440, 273–282. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Palazzo, F.; Di Michele, A.; Schoubben, A.; Blasi, P.; Ricci, M. The influence of feedstock and process variables on the encapsulation of drug suspensions by spray-drying in fast drying regime: The case of novel antitubercular drug-palladium complex containing polymeric microparticles. J. Pharm. Sci. 2014, 103, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, S.; Pietrella, D.; Barberini, L.; Santi, C.; Carotti, A.; di Michele, A.; Ricci, M. Reshaping antibiotics through hydrophobic drug-bile acid ionic complexation enhances activity against Staphylococcus aureus biofilms. Int. J. Pharm. 2017, 528, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Tolmasky, M. Amikacin: Uses, Resistance, and Prospects for Inhibition. Molecules 2017, 22, 2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clancy, J.P.; Dupont, L.; Konstan, M.W.; Billings, J.; Fustik, S.; Goss, C.H.; Lymp, J.; Minic, P.; Quittner, A.L.; Rubenstein, R.C.; et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013, 68, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, I.; Wallet, F.; Robert, J.; Becquemin, M.H.; Marquette, C.H.; Rouby, J.J. Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am. J. Respir. Crit. Care Med. 2002. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.J.; Neville, M.E.; Gupta, R.; Bermudez, L.E. Delivery of Aerosolized Liposomal Amikacin as a Novel Approach for the Treatment of Nontuberculous Mycobacteria in an Experimental Model of Pulmonary Infection. PLoS ONE 2014, 9, e108703. [Google Scholar] [CrossRef] [Green Version]
- Luyt, C.-E.; Eldon, M.A.; Stass, H.; Gribben, D.; Corkery, K.; Chastre, J. Pharmacokinetics and Tolerability of Amikacin Administered as BAY41-6551 Aerosol in Mechanically Ventilated Patients with Gram-Negative Pneumonia and Acute Renal Failure. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 183–190. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Russo, A.; Peghin, M. Inhaled Liposomal Antimicrobial Delivery in Lung Infections. Drugs 2020, 80, 1309–1318. [Google Scholar] [CrossRef]
- Swenson, C.; Lapinel, N.C.; Ali, J. Clinical management of respiratory adverse events associated with amikacin liposome inhalation suspension: Results from a patient survey. Open Forum Infect. Dis. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Winthrop, K.; Morimoto, K.; Castellotti, P.F.; Yim, J.-J.; Ruoss, S.; van Ingen, J.; Coulter, C.; Mange, K.; Nezamis, J.; Griffith, D. An open-label extension study of amikacin liposome inhalation suspension (ALIS) for treatment-refractory lung disease caused by mycobacterium avium complex (MAC). Chest 2019, 156, A146–A147. [Google Scholar] [CrossRef]
- Griffith, D.E.; Eagle, G.; Thomson, R.; Aksamit, T.R.; Hasegawa, N.; Morimoto, K.; Addrizzo-Harris, D.J.; O’Donnell, A.E.; Marras, T.K.; Flume, P.A.; et al. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT). A Prospective, Open-Label, Randomized Study. Am. J. Respir. Crit. Care Med. 2018, 198, 1559–1569. [Google Scholar] [CrossRef]
- Griffith, D.E.; Thomson, R.; Addrizzo-Harris, D.J.; Field, S.K.; van Ingen, J.; Wallace, R.J., Jr.; Coulter, C.; Mange, K.; Nezamis, J.; Winthrop, K.L. Sustainability and Durability of Culture Conversion in Patients Receiving Amikacin Liposome Inhalation Suspension (ALIS) for Treatment-Refractory Mycobacterium Avium Complex Lung Disease (MAC-LD) in the CONVERT Study. In Proceedings of the B14. Late Breaking Clinical Trials, Dallas, TX, USA, 17–22 May 2019; p. A7359. [Google Scholar]
- Brown-Elliott, B.A.; Eagle, G.; Wallace, R.J.; Van Ingen, J.; Pennings, L.J.; Berry, B.; Pandey, S.; Coulter, C.; Syrmis, M.; Winthrop, K.L.; et al. 805. Amikacin Liposome Inhalation Suspension (ALIS) Add-on Therapy for Refractory Mycobacterium avium Complex (MAC) Lung Disease: Effect of In Vitro Amikacin Susceptibility on Sputum Culture Conversion. Open Forum Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghaffari, S.; Mirshojaei, S.F.; Jafarian, A.; Atyabi, F.; Kobarfard, F.; Azarmi, S. Biodistribution of amikacin solid lipid nanoparticles after pulmonary delivery. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetényi, G.; Griesser, J.; Fontana, S.; Gutierrez, A.M.; Ellemunter, H.; Niedermayr, K.; Szabó, P.; Bernkop-Schnürch, A. Amikacin-containing self-emulsifying delivery systems via pulmonary administration for treatment of bacterial infections of cystic fibrosis patients. Nanomedicine 2018. [Google Scholar] [CrossRef]
- Shirley, M. Amikacin liposome inhalation suspension: A review in Mycobacterium avium complex lung disease. Drugs 2019, 79, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Khan, O.; Chaudary, N. The use of amikacin liposome inhalation suspension (Arikayce) in the treatment of refractory nontuberculous mycobacterial lung disease in adults. Drug Des. Dev. Ther. 2020, 14, 2287–2294. [Google Scholar] [CrossRef]
- Nicoli, S.; Santi, P. Assay of amikacin in the skin by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2006, 41, 994–997. [Google Scholar] [CrossRef]
- Papp, E.A.; Knupp, C.A.; Barbhaiya, R.H. High-performance liquid chromatographic assays for the quantification of amikacin in human plasma and urine. J. Chromatogr. B Biomed. Sci. Appl. 1992, 574, 93–99. [Google Scholar] [CrossRef]
- Bailer, A.J. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokinet. Biopharm. 1988. [Google Scholar] [CrossRef]
- Gagnon, R.C.; Peterson, J.J. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J. Pharmacokinet. Biopharm. 1998, 26, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, Z.; Wetzel, J.D.; Clancy, J.P. Nebulized liposomal amikacin for the treatment of Pseudomonas aeruginosa infection in cystic fibrosis patients. Expert Opin. Investig. Drugs 2014, 23, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Redington, J.; Ebert, S.C. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 1991, 27, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Margetts, P.J.; Anthony, D.C.; Pitossi, F.; Gauldie, J. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Investig. 2001, 107, 1529–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, R.; Janssen, W.; Altmann, C.; Andrés-Hernando, A.; Okamura, K.; Vandivier, R.W.; Ahuja, N.; Faubel, S. Intratracheal IL-6 protects against lung inflammation in direct, but not indirect, causes of acute lung injury in mice. PLoS ONE 2013, 8, e61405. [Google Scholar] [CrossRef] [PubMed]
- Voiriot, G.; Razazi, K.; Amsellem, V.; Tran Van Nhieu, J.; Abid, S.; Adnot, S.; Mekontso Dessap, A.; Maitre, B. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Natalini, P.M.; Razuc, M.F.; Sørli, J.B.; Bucalá, V.; Ramírez-Rigo, M.V. The influence of surfactant on the properties of albendazole-bile salts particles designed for lung delivery. J. Drug Deliv. Sci. Technol. 2019, 53, 101162. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Danić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Nakada, E.M.; Bhakta, N.R.; Korwin-Mihavics, B.R.; Kumar, A.; Chamberlain, N.; Bruno, S.R.; Chapman, D.G.; Hoffman, S.M.; Daphtary, N.; Aliyeva, M.; et al. Conjugated bile acids attenuate allergen-induced airway inflammation and hyperresposiveness by inhibiting UPR transducers. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- French, M.A.; Cerra, F.B.; Plaut, M.E.; Schentag, J.J. Amikacin and gentamicin accumulation pharmacokinetics and nephrotoxicity in critically ill patients. Antimicrob. Agents Chemother. 1981. [Google Scholar] [CrossRef] [Green Version]
- Schentag, J.J.; Jusko, W.J.; Plaut, M.E.; Cumbo, T.J.; Vance, J.W.; Abrutyn, E. Tissue Persistence of Gentamicin in Man. JAMA J. Am. Med. Assoc. 1977. [Google Scholar] [CrossRef]
- Alonso, I.G.; Lanao, J.M.; Saez, M.C.; Dominguez-Gil, A.A.; Dominguez-Gil, A. Non-linear tissue binding of amikacin in rats: The effect of renal impairment. Eur. J. Drug Metab. Pharmacokinet. 1987. [Google Scholar] [CrossRef] [PubMed]
- Kornguth, M.L.; Kunin, C.M. Distribution of Gentamicin and Amikacin in Rabbit Tissues. Antimicrob. Agents Chemother. 1977, 11, 974–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.G.; Chen, M.-L.; Huang, S.-M.; Chiou, W.L. Pharmacokinetics of drugs in blood I. Unusual distribution of gentamicin. Biopharm. Drug Dispos. 1981. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Amk Solution | Amk-DCA Suspension | ||
|---|---|---|---|---|
| Lung | Blood | Lung | Blood | |
| Cmax (μg/mL or μg/g) | 66.0 ± 10.4 | 6.4 ± 1.7 | 41.3 ± 7.1 | 6.1 ± 0.5 |
| Tmax (h) | 0.5 | 0.5 | 0.5 | 0.5 |
| AUC 0–48h (μg h/mL or μg h/g) | 1204.0 ± 76.2 | 219.6 ± 44.1 | 1275.0 ± 126.8 | 217.5 ± 22.3 |
| AUC lung to AUC blood ratio | 5.6 ± 1.1 | 5.9 ± 1.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiroudaki, S.; Ianni, F.; Sabbatini, S.; Roselletti, E.; Monari, C.; Sardella, R.; Vecchiarelli, A.; Giovagnoli, S. Initial In Vivo Evaluation of a Novel Amikacin-Deoxycholate Hydrophobic Salt Delivers New Insights on Amikacin Partition in Blood and Tissues. Pharmaceutics 2021, 13, 85. https://doi.org/10.3390/pharmaceutics13010085
Xiroudaki S, Ianni F, Sabbatini S, Roselletti E, Monari C, Sardella R, Vecchiarelli A, Giovagnoli S. Initial In Vivo Evaluation of a Novel Amikacin-Deoxycholate Hydrophobic Salt Delivers New Insights on Amikacin Partition in Blood and Tissues. Pharmaceutics. 2021; 13(1):85. https://doi.org/10.3390/pharmaceutics13010085
Chicago/Turabian StyleXiroudaki, Styliani, Federica Ianni, Samuele Sabbatini, Elena Roselletti, Claudia Monari, Roccaldo Sardella, Anna Vecchiarelli, and Stefano Giovagnoli. 2021. "Initial In Vivo Evaluation of a Novel Amikacin-Deoxycholate Hydrophobic Salt Delivers New Insights on Amikacin Partition in Blood and Tissues" Pharmaceutics 13, no. 1: 85. https://doi.org/10.3390/pharmaceutics13010085