Abstract
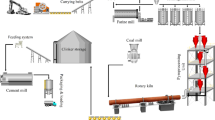
The most energy demanding process in the cement industry is clinker production, carried out in a rotary kiln. Thus, rotary kiln energetic and exergetic analyses are useful tools to reach cement production process improvements. Energetic analysis is based on the first law of thermodynamics and allows one to calculate the heat uses and losses. On the other hand, exergetic analysis is based on a combination of the First and Second Laws of Thermodynamics and allows quantifying process irreversibilities. Some rotary kiln exergy analyses neglect the mass flows chemical exergy in the exergetic analysis, considering only the fuel chemical exergy. In this work, chemical exergy impact on this analysis was evaluated. Pre-calcination effect was also studied. Rotary kiln classical and modern exergetic efficiency considering all the chemical exergy contributions were 55.5% and 41.8%, respectively, while considering just the fuel chemical exergy were 38.2% and 22.6%, respectively. The results showed that it is inadequate to neglect the mass flows chemical exergy, since their contribution in the exergetic analysis was relevant. Furthermore, it was observed that the method adopted in the process efficiency evaluation affects the system interpretation. Considering the modern exergetic efficiency, results showed that, the higher the pre-calcination, the higher rotary kiln efficiency.

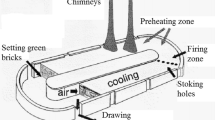
adapted from Giannopoulos et al. (2007))












Similar content being viewed by others
Abbreviations
- A:
-
Fuel mass percentage of ash in dry basis
- \({c_i}\) :
-
Element fraction appearing in the form of the reference species.
- \({c_p}\) :
-
Heat capacity at constant pressure (J/mol.K)
- C:
-
Fuel mass percentage of carbon in dry basis.
- \(E\) :
-
Energy (kJ)
- \(\dot E\) :
-
Energy rate (kJ/h)
- \(ex\) :
-
Specific exergy (J/mol)
- \(ex_{ch}^0\) :
-
Specific standard chemical exergy (J/mol)
- \(Ex\) :
-
Exergy (kJ)
- \({\dot {E}}\) :
-
Exergy rate (kJ/h)
- g:
-
Gravitational acceleration (m/s2)
- \(g_f^0\) :
-
Standard Gibbs energy of formation (J/mol)
- \(h\) :
-
Specific Enthalpy (J/mol)
- \(h_f^0\) :
-
Specific Enthalpy of formation (J/mol)
- H:
-
Fuel mass percentage of hydrogen in dry basis.
- j:
-
Number of reference ions or molecules derived from one molecule of the element under consideration.
- \({l_i}\) :
-
Number of atoms of the element in the molecule of the reference species.
- \(\dot m\) :
-
Mass flow rate (kg/h)
- \({M_0}\) :
-
Lithosphere molecular mass (kg/mol)
- \({n_{i,0}}\) :
-
Lithosphere molar concentration of the ith element.
- N:
-
Fuel mass percentage of Nitrogen in dry basis.
- \({N_k}\) :
-
Number of molecules of additional elements present in the molecule of the reference species.
- O:
-
Fuel mass percentage of oxygen in dry basis.
- P:
-
Pressure (atm)
- pH:
-
Negative logarithm of the hydrogen ion concentration.
- \(\dot Q\) :
-
Heat transfer rate (kJ/h)
- R:
-
Universal gas constant. (J/mol.K)
- s:
-
Specific Entropy (J/mol.K)
- S:
-
Coal mass percentage of sulfur in dry basis.
- \(T\) :
-
Temperature (K)
- \({u_0}\) :
-
Conventional standard molar concentration of the reference species in seawater.
- v:
-
Velocity (m/s)
- \(\dot W\) :
-
Power (W)
- x:
-
Mass fraction.
- z:
-
Position
- \(\alpha\) :
-
Heat capacity at constant pressure parameter. (*)
- \(\beta\) :
-
Heat capacity at constant pressure parameter. (*)
- \(\delta\) :
-
Heat capacity at constant pressure parameter. (*)
- \(\varepsilon\) :
-
Heat capacity at constant pressure parameter. (*)
- \(\gamma\) :
-
Activity coefficient.
- \({\eta_{en}}\) :
-
Energetic Efficiency.
- \({\eta_{ex}}\) :
-
Exergetic Efficiency.
- \(\mu\) :
-
Chemical potential. (J/mol)
- \(\sigma\) :
-
Heat capacity at constant pressure parameter. (*)
- \(\nu\) :
-
Stoichiometric coefficient.
- \({\chi_i}\) :
-
Reference specie mole fraction in the lithosphere
- *:
-
The heat capacity at constant pressure parameters have different units as a function of the heat capacity equation. For each equation, the parameters units are defined in such a way that the heat capacity has the unit (J/mol.K).
- 0:
-
Restricted dead state.
- 00:
-
Absolute dead state.
- ch:
-
Chemical.
- i:
-
Specie.
- r:
-
Reaction.
- d:
-
Destroyed.
- el:
-
Element.
References
Anacleto TF, Turetta LF, Jr C, EF; Costa, AOS, (2018) Efeito da reação de calcinação nas análises energética e exergética de um forno rotativo empregado na produção de clínquer. Cerâmica 64:507–518. https://doi.org/10.1590/0366-69132018643722416
Araújo ACB, Vasconcelos LGS, Fossy MF, Brito RP (2007) Exergetic and economic analysis of an industrial distillation column. Braz J Chem Eng 24:461–469. https://doi.org/10.1590/S0104-66322007000300015
Atmaca A, Yumrutas R (2014) Analysis of the parameters affecting energy consumption of a rotary kiln in cement industry. Appl Therm Eng 64:435–444. https://doi.org/10.1016/j.applthermaleng.2014.02.038
Atmaca A, Yumrutaş R (2014a) Thermodynamic and exergoeconomic analysis of a cement plant: Part i - Methodology. Energy Convers Manage 79:790–798. https://doi.org/10.1016/j.enconman.2013.11.053
Atmaca A, Yumrutaş R (2014b) Thermodynamic and exergoeconomic analysis of a cement plant: Part ii - Application. Energy Convers Manag 79:799–808. https://doi.org/10.1016/j.enconman.2013.11.054
Bühler F, Nguyen T, Kjær J, Müller F, Elmegaard B (2018) Energy, exergy and advanced exergy analysis of a milk processing factory. Energy 162:576–592. https://doi.org/10.1016/j.energy.2018.08.029
Caglayan H (2018) Caliskan, H (2018) Energy, exergy and sustainability assessments of a cogeneration system for ceramic industry. Appl Therm Eng 136:504–515. https://doi.org/10.1016/j.applthermaleng.2018.02.064
Çamdali Ü, Erişen A, Çelen F (2004) Energy and exergy analyses in a rotary burner with pre-calcinations in cement production. Energy Convers Manag 45:3017–3031. https://doi.org/10.1016/j.enconman.2003.12.002
Cembureau, KEY FACTS & FIGURES / KEY FACTS. https://cembureau.eu/cement-101/key-facts-figures/. Accessed 02 April 2020
Costa VAF (2016) On the exergy balance equation and the exergy destruction. Energy 116:824–835. https://doi.org/10.1016/j.energy.2016.10.015
Darvishi H (2017) Quality performance analysis, mass transfer parameters and modeling of drying kinetics of soybean. Braz J Chem Eng 34:143–158
Fellaou S, Bounahmidi T (2017) Evaluation of energy efficiency opportunities of a typical Moroccan cement plant: part I. Energy Anal Appl Therm Eng 115:1161–1172. https://doi.org/10.1016/j.applthermaleng.2017.01.010
Fellaou S, Bounahmidi T (2018) Analyzing thermodynamic improvement potential of a selected cement manufacturing process: advanced exergy analysis. Energy 154:190–200. https://doi.org/10.1016/j.energy.2018.04.121
Fellaou S, Harnoune A, Seghra MA, Bounahmidi T (2018) Statistical modeling and optimization of the combustion efficiency in cement kiln precalciner. Energy 155:351–359. https://doi.org/10.1016/j.energy.2018.04.181
Fraga MMC, De Campos BLO, Lisboa MS, De Almeida TB, Da Costa AOS, De Lins VFC (2018) Analysis of a Brazilian thermal plant operation applying energetic and exergetic balances. Braz J Chem Eng 35:1395–1403. https://doi.org/10.1590/0104-6632.20180354s20170425
Gharagheizi F, Ilani-Kashkouli P, Mohammadi AH, Ramjugernath D (2014) A group contribution method for determination of the standard molar chemical exergy of organic compounds. Energy 70:288–297. https://doi.org/10.1016/j.energy.2014.03.124
Giannopoulos D, Kolaitis DI, Togkalidou A, Skevis G, Founti MA (2007) Quantification of emissions from the co-incineration of cutting oil emulsions in cement plants: Part II: Trace species. Fuel 86:2491–2501. https://doi.org/10.1016/j.fuel.2006.10.025
Gürtürk M, Oztop HF (2014) Energy and exergy analysis of a rotary kiln used for plaster production. Appl Therm Eng 67:554–565. https://doi.org/10.1016/j.applthermaleng.2014.03.025
Jung IH, Hudon P (2012) Thermodynamic assessment of P2O5. J Am Ceram Soc 95:3665–3672. https://doi.org/10.1111/j.1551-2916.2012.05382.x
Kabir G, Abubakar AI, El-Nafaty UA (2010) Energy audit and conservation opportunities for pyroprocessing unit of a typical dry process cement plant. Energy 35:1237–1243. https://doi.org/10.1016/j.energy.2009.11.003
Lazzaretto A, Tsatsaronis G (2006) SPECO: A systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy 31:1257–1289. https://doi.org/10.1016/j.energy.2005.03.011
Lothenbach B, Kulik DA, Matschei T, Balonis M, Baquerizo L, Dilnesa B, Miron GD, Myers RJ (2019) Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem Concr Res 115:472–506. https://doi.org/10.1016/j.cemconres.2018.04.018
Madlool NA, Saidur R, Rahim NA, Islam MR, Hossian MS (2012) An exergy analysis for cement industries: An overview. Renew Sustain Energy Rev 16:921–932. https://doi.org/10.1016/j.rser.2011.09.013
Mastral F, Ceamanos J, Atienza-martínez M, Abrego J, Gea G (2018) Energy and exergy analyses of sewage sludge thermochemical treatment. Energy 144:723–735. https://doi.org/10.1016/j.energy.2017.12.007
Matschei T, Lothenbach B, Glasser FP (2007) Thermodynamic properties of Portland cement hydrates in the system CaO-Al2O3-SiO2-CaSO4-CaCO3-H2O. Cem Concr Res 37:1379–1410. https://doi.org/10.1016/j.cemconres.2007.06.002
Nat. Inst. Stand. Tech. Pesquisa para dados de espécies através da fórmula química. https://webbook.nist.gov/chemistry/form-ser/. Accessed 02 April 2020.
Ortega-Delgado B, Giacalone F, Catrini P, Cipollina A, Piacentino A, Tamburini A, Micale G (2019) Reverse electrodialysis heat engine with multi-effect distillation: Exergy analysis and perspectives. Energy Convers Manag 194:140–159. https://doi.org/10.1016/j.enconman.2019.04.056
Özilgen M (2018) Nutrition and production related energies and exergies of foods. Renew Sustain Energy Rev 96:275–295. https://doi.org/10.1016/j.rser.2018.07.055
Qian H, Zhu W, Fan S, Liu C, Lu X, Wang Z, Huang D, Chen W (2017) Prediction models for chemical exergy of biomass on dry basis from ultimate analysis using available electron concepts. Energy 131:251–258. https://doi.org/10.1016/j.energy.2017.05.037
Querino MV, Machado RAF, Marangoni C (2019) Energy and exergetic evaluation of the multicomponent separation of petrochemical naphtha in falling film distillation columns. Braz J Chem Eng 36:1357–1365. https://doi.org/10.1590/0104-6632.20190363s20180379
Ramos VF, Pinheiro OS, Ferreira da Costa E, Souza da Costa AO (2019) A method for exergetic analysis of a real kraft biomass boiler. Energy 183:946–957. https://doi.org/10.1016/j.energy.2019.07.001
Renó MLG, Torres FM, Da Silva RJ, Santos JJCS, Melo MDLNM (2013) Exergy analyses in cement production applying waste fuel and mineralizer. Energy Convers Manag 75:98–104. https://doi.org/10.1016/j.enconman.2013.05.043
Rivero R, Garfias M (2006) Standard chemical exergy of elements updated. Energy 31:3310–3326. https://doi.org/10.1016/j.energy.2006.03.020
Shahin H, Hassanpour S, Saboonchi A (2016) Thermal energy analysis of a lime production process: Rotary kiln, preheater and cooler. Energy Convers Manag 114:110–121. https://doi.org/10.1016/j.enconman.2016.02.017
Smith JM, Van Ness HC, Abbott MM (2007) Introdução à Termodinâmica da Engenharia Química. 7. ed. LTC.
Som SK, Datta A (2008) Thermodynamic irreversibilities and exergy balance in combustion processes. Prog Energy Combust Sci 34:351–376. https://doi.org/10.1016/j.pecs.2007.09.001
Song G, Xiao J, Zhao H, Shen L (2012) A unified correlation for estimating specific chemical exergy of solid and liquid fuels. Energy 40:164–173. https://doi.org/10.1016/j.energy.2012.02.016
Szargut J (1989) Chemical Exergies of the Elements. Appl Energy 32:269–286. https://doi.org/10.1016/0306-2619(89)90016-0
Terehovics E, Veidenbergs I, Blumberga D (2017) Energy and exergy balance methodology Wood chip dryer. Energy Procedia 128:551–557. https://doi.org/10.1016/j.egypro.2017.09.008
Thermal energy consumption—weighted average. (2016). https://www.wbcsdcement.org/GNR-2016/world/GNR-Indicator_93DWGck-world-2016.html. Accessed 02 April 2020
Ustaoglu A, Alptekin M, Akay ME (2017) Thermal and exergetic approach to wet type rotary kiln process and evaluation of waste heat powered ORC (organic Rankine cycle). Appl Therm Eng 112:281–295. https://doi.org/10.1016/j.applthermaleng.2016.10.053
Vučković GD, Stojiljković MM, Vukić MV, Stefanović GM, Dedeić EM (2014) Advanced exergy analysis and exergoeconomic performance evaluation of thermal processes in an existing industrial plant. Energy Convers Manag 85:655–662. https://doi.org/10.1016/j.enconman.2014.03.049
Yildirim N, Genc S (2017) Energy and exergy analysis of a milk powder production system. Energy Convers Manag 149:698–705. https://doi.org/10.1016/j.enconman.2017.01.064
Zhao Y, Wang S, Ge M, Li Y, Yang Y (2018) Energy and exergy analysis of thermoelectric generator system with humidified flue gas. Energy Convers Manag 156:140–149. https://doi.org/10.1016/j.enconman.2017.10.094
Acknowledgements
The authors are grateful to CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico, Capes—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and FAPEMIG—Fundação de Amparo à Pesquisa do Estado de Minas Gerais (TEC-APQ-00914-16) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anacleto, T.F., e Silva, A.E.G.d., da Silva, S.R. et al. Chemical exergy influence in the exergetic analysis of a real clinker rotary kiln. Braz. J. Chem. Eng. 38, 197–214 (2021). https://doi.org/10.1007/s43153-020-00084-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00084-0