Abstract
This paper discusses the effects of composition on the unit cell parameter (a), unit cell volume (V), specific volume (v), and density (ρ) of various sodalites including \({M}_{8}^{+}{\left({\mathrm{AlSiO}}_{4}\right)}_{6}{\mathrm{Cl}}_{2}\) (M = Li, Na, K, Rb, and/or Ag) and \({\mathrm{Na}}_{8}{\left({\mathrm{AlSiO}}_{4}\right)}_{6}{X}_{2}^{-}\) (X = Cl, Br, and/or I). Compositional models were developed, and the results show that the models are successful at predicting a, V, and v (and thus ρ) within the compositional range available in the literature. Discussion is included on the correlation between the ionic radii of the alkali metals and halides in the sodalite β-cages and the measured values of a, V, v, and ρ. The data show linear increases in a and ρ with increases in the average ionic radii of the \({M}^{+}\) and \({X}^{-}\) constituents (data for v show a linear decrease).

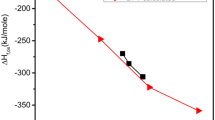
modified from a previous figure by Riley et al. (2016b) and reprinted with permission. ©2016 Elsevier




Similar content being viewed by others
References
Antao SM, Hassan I, Wang J, Lee PL, Toby BH (2008) State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can Mineral 46(6):1501–1509
Barrer RM, Vaugiian DEW (1971) Trapping of inert gases in sodalite and cancrinite crystals. J Phys Chem Solids 32(3):731–743
Barth TFW (1932) The structures of the minerals of the sodalite family. Z Kristallogr Kristallgeom Kristallphys Kristallchem. 83:405–414
Bateman KJ, Knight CJ, Solbrig CW (2007) Current status of ceramic waste form development. INL/INT-06–11736, Rev. 1, Idaho National Laboratory, Idaho Falls, ID
Beagley B, Henderson CMB, Taylor D (1982) The crystal structures of aluminoslicate-sodalites: X-ray diffraction studies and computer modelling. Mineral Mag 46:459–464
Boocock SK (2009) Non-chromate corrosion inhibitor formulas based on permanganate sodalite compositions.USA Patent No. US 2009/0075113 A1
Borhade AV, Wakchaure SG, Dholi AG (2010) One pot synthesis and crystal structure of aluminosilicate mixed chloro-iodo sodalite. Indian J Phys 84(2):133–141
Chong S, Riley BJ, Asmussen RM, Lawter AR, Bruffey SH, Nam J, McCloy JS, Crum JV (2020) Iodosodalite synthesis with hot isostatic pressing: a comparison between aqueous and hydrothermal synthesis processes. J Nucl Mater 538:152222
Cotton FA, Wilkinson G (1980) Advanced inorganic chemistry: a comprehensive text, 4th edn. Wiley Interscience, New York, NY
Frank S, Barber T, Lambregts M (2005) Powder diffraction of sodalite in a multiphase ceramic used to immobilize radioactive waste. Powder Diffr 20(3):212–214
Hassan I, Grundy HD (1984) The crystal structures of sodalite-group minerals. Acta Crystallogr B 40(1):6–13
Hassan I, Antao SM, Parise JB (2004) Sodalite: high-temperature structures obtained from synchrotron radiation and Rietveld refinements. Am Miner 89:359–364
Henderson CMB, Taylor D (1977) Infrared spectra of anhydrous members of the sodalite family. Spectrochim Acta 33A:283–290
Henderson CMB, Taylor D (1978) The thermal expansion of synthetic aluminosilicate-sodalites, M8(Al6Si6O24)X2. Phys Chem Mater 2:337–347
Jansen CJ, Kapteijn F, Khajavi S (2011) Process for the production of ultra pure water using a membrane. Netherlands Application No. EP2192090 A1
Koyama T (1994) Method to synthesize dense crystallized sodalite pellet for immobilizing halide salt radioactive waste. United States Patent No. 5340506.
Kroll JO, Riley BJ, McCloy JS, Peterson JA (2020) Sol-gel synthesis of iodosodalite and subsequent consolidation with a glass binder made from oxides and sol-gel routes. J Sol-Gel Sci Technol 96:564–575
Lacks DJ, Gordon RG (1993) Crystal-structure calculations with distorted ions. Phys Rev B 48(5):2889–2908
Lepry WC, Riley BJ, Crum JV, Rodriguez CP, Pierce DA (2013) Solution-based approaches for making high-density sodalite waste forms to immobilize spent electrochemical salts. J Nucl Mater 442(1–3):350–359
Löns J, Schulz H (1967) Strukturverfeinerung von Sodalith, Na8Si6Al6O24Cl2. Acta Crystallogr A 23(3):434–436
Marsh A, Heath A, Patureau P, Evernden M, Walker P (2018) A mild conditions synthesis route to produce hydrosodalite from kaolinite, compatible with extrusion processing. Microporous Mesoporous Mater 264:125–132
Murshed MM, Gesing TM (2007) Isomorphous gallium substitution in the alumosilicate sodalite framework: synthesis and structural studies of chloride and bromide containing phases. Zeitschrift fuer Kristallographie 222(7):341–349
Nielsen NC, Bildsøe H, Jakobsen HJ, Norby P (1991) 7Li, 23Na, and 27Al quadrupolar interactions in some aluminosilicate sodalites from MAS NMR spectra of satellite transitions. Zeolites 11(6):622–632
Oliveira Siqueira G, Goncalves Gravina E, Lamounier Camargos Resende JA, Goncalves Fernandes N (2009) XRD diffraction data and Rietveld refinement of Na8(Si6Al6O24)Cl2. Powder Diffr 24(1):41–43
Pauling L (1930) The structure of sodalite and helvite. Z Kristallogr Kristallgeom Kristallphys Kristallchem 74:213–225
Peterson RC (1983) The structure of hackmanite, a variety of sodalite, from Mont St-Hilaire, Quebec. Can Mineral 21(3):549–552
Riley BJ, Pierce DA, Frank SM, Matyáš J, Burns CA (2015) Efficacy of a solution-based approach for making sodalite waste forms for an oxide reduction salt used in the reprocessing of used uranium oxide fuel. J Nucl Mater 459:313–322
Riley BJ, Lepry WC, Crum JV (2016) Solution-derived sodalite made with Si- and Ge-ethoxide precursors for immobilizing electrorefiner salt. J Nucl Mater 468:140–146
Riley BJ, Vienna JD, Strachan DM, McCloy JS, Jerden JL Jr (2016) Materials and processes for the effective capture and immobilization of radioiodine: a review. J Nucl Mater 470:307–326
Riley BJ, Vienna JD, Frank SM, Kroll JO, Peterson JA, Canfield NL, Zhu Z, Zhang J, Kruska K, Schreiber DK, Crum JV (2017) Glass binder development for a glass-bonded sodalite ceramic waste form. J Nucl Mater 489:42–63
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A A32(5):751–767
Stein A, Ozin GA, Macdonald PM, Stucky GD, Jelinek R (1992) Silver, sodium halosodalites: class A sodalites. J Am Chem Soc 114(13):5171–5186
Sugdon MC, Weller MT (2005) Security material. United Kingdom Application No. EP1681335 A2
Tamazyan RA, Malinovskii YA, Il’inets AM (1988) Atomic structure and microtwinning of sodalite. Sov Phys Crystallogr 33(3):325–329
Taylor D, Henderson CMB (1978) A computer model for the cubic sodalite structure. Phys Chem Minerals 2:325–336
Vance ER, Davis J, Olufson K, Chironi I, Karatchevtseva I, Farnan I (2012) Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. J Nucl Mater 420(1–3):396–404
Vegard L (1921) Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Zeitschrift für Physik 5(1):17–26
Wartchow R (1997) Redetermination of the crystal structure of hexaaluminium hexasilicon octasodium dichloride tetracosaoxide (sodalite) Na8(Al6Si6O24)Cl2. Zeitschrift fuer Kristallographie—New Crystal Structures 212(2):80
Weller MT, Wong G (1989a) Characterisation of novel sodalites by neutron diffraction and solid-state NMR. Solid State Ion 32–33(1):430–435
Weller MT, Wong G (1989b) Mixed halide sodalites. Eur J Solid State Inorg Chem 26(6):619–633
Zahoransky T, Friis H, Marks MAW (2016) Luminescence and tenebrescence of natural sodalites: a chemical and structural study. Phys Chem Miner 43(7):459–480
Acknowledgements
Pacific Northwest National Laboratory (PNNL) is operated by Battelle Memorial Institute for the DOE under contract DE-AC05-76RL01830. This work was supported by the DOE Office of Nuclear Energy (DOE-NE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riley, B.J., Peterson, J.A., Chong, S. et al. Influence of ion site occupancies on the unit cell parameters, specific volumes, and densities of M8(AlSiO4)6X2 sodalites where M = Li, Na, K, Rb, and Ag and X = Cl, Br, and I. Phys Chem Minerals 48, 3 (2021). https://doi.org/10.1007/s00269-020-01124-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-020-01124-4