Abstract
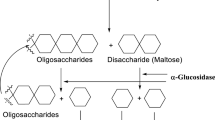
In this study, a new series of quinazolinone-1,2,3-triazole-acetamide hybrids 8a–m, using by molecular hybridization of the potent α-glucosidase inhibitor pharmacophores, was designed and evaluated against carbohydrate-hydrolyzing enzymes α-glucosidase and α-amylase. All the synthesized compounds with IC50 values in the range of 45.3 ± 1.4 µM to 195.5 ± 4.7 µM were significantly more potent than standard inhibitor against α-glucosidase, while these compounds were not active against α-amylase in comparison to standard inhibitor. Representatively, compound 8a with IC50 = 45.3 ± 1.4 µM was around 17 times more potent than standard inhibitor acarbose (IC50 = 750.0 ± 12.5 µM). The inhibition kinetic analysis of the compound 8a indicated that this compound was a competitive α-glucosidase inhibitor. Molecular modeling analysis confirmed that the most potent inhibitors 8a and 8b well accommodated in the modeled α-glucosidase active site and it was also revealed that these compounds formed stable inhibitor–receptor complexes with the α-glucosidase in comparison to acarbose. In silico pharmacokinetic and toxicity of the most potent compounds were evaluated and obtained results were compared with acarbose. Furthermore, the most potent compounds were also evaluated against human normal cells and no cytotoxicity was observed.







Similar content being viewed by others
References
Heine RJ, Diamant M, Mbanya JC, Nathan DM. Management of hyperglycaemia in type 2 diabetes: the end of recurrent failure? BMJ. 2006;333:1200–4.
Yang CY, Yen YY, Hung KC, Hsu SW, Lan SJ, Lin HC. Inhibitory effects of pu-erh tea on alpha glucosidase and alpha amylase: a systemic review. Nutr Diabetes. 2019;9:1–6.
Power RF. Enzymatic conversion of starch to fermentable sugars. The Alcohol Textbook, Ethanol Technology Institute, Duluth, Georgia. 2003;23–32.
Derosa G, Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci. 2012;8:899.
Kim MJ, Lee SB, Lee HS, Lee SY, Baek JS, Kim D, et al. Comparative study of the inhibition of α-glucosidase, α-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine–glucose. Arch Biochem Biophys. 1999;371:277–83.
Dong Y, Zhang B, Sun W, Xing Y. Intervention of Prediabetes by Flavonoids From Oroxylum indicum. In Bioactive Food as Dietary Interventions for Diabetes: Academic Press, Elsevier, Cambridge, Massachusetts, 559;2019.
Ma CM, Hattori M, Daneshtalab M, Wang L. Chlorogenic acid derivatives with alkyl chains of different lengths and orientations: potent α-glucosidase inhibitors. J Med Chem. 2008;51:6188–94.
Wos M, Miazga-Karska M, Kaczor AA, Klimek K, Karczmarzyk Z, Kowalczuk D, et al. Novel thiosemicarbazide derivatives with 4-nitrophenyl group as multi-target drugs: α-glucosidase inhibitors with antibacterial and antiproliferative activity. Biomed Pharmacother. 2017;93:1269–76.
Channar PA, Saeed A, Larik FA, Rashid S, Iqbal Q, Rozi M, et al. Design and synthesis of 2,6-di(substituted-phenyl)thiazolo[3,2-b]-1,2,4-triazoles as αglucosidase and α-amylase inhibitors, co-relative pharmacokinetics and 3D QSAR and risk analysis. Biomed Pharmacother. 2017;94:499–513.
Bohacek RS, McMartin C, Guida WC. The art and practice of structure‐based drug design: a molecular modeling perspective. Med Res Rev. 1996;16:3–50.
Santos CM, Freitas M, Fernandes E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur J Med Chem. 2018;157:1460–79.
Rajput R, Mishra AP. A review on biological activity of quinazolinones. Int J Pharm Pharm Sci. 2012;4:66–70.
Mohammadi‐Khanaposhtani M, Yahyavi H, Imanparast S, Harandi FN, Faramarzi MA, Foroumadi A, et al. Benzoylquinazolinone derivatives as new potential antidiabetic agents: α‐glucosidase inhibition, kinetic, and docking studies. J Chin Chem Soc. 2020;67:856–63.
Wei M, Chai WM, Wang R, Yang Q, Deng Z, Peng Y. Quinazolinone derivatives: synthesis and comparison of inhibitory mechanisms on α-glucosidase. Bioorg Med Chem. 2017;25:1303–8.
Mohammadi-Khanaposhtani M, Rezaei S, Khalifeh R, Imanparast S, Faramarzi MA, Bahadorikhalili S, et al. Design, synthesis, docking study, α-glucosidase inhibition, and cytotoxic activities of acridine linked to thioacetamides as novel agents in treatment of type 2 diabetes. Bioorg Chem. 2018;80:288–95.
Mohammadi-Khanaposhtani M, Yahyavi H, Barzegaric E, Imanparast S, Heravi MM, Faramarzi MA, et al. New biscoumarin derivatives as potent α-glucosidase inhibitors: synthesis, biological evaluation, kinetic analysis, and docking study. Polycycl Aromat Comp. 2018;40:915–26.
Asemanipoor N, Mohammadi-Khanaposhtani M, Moradi S, Vahidi M, Asadi M, Faramarzi MA, et al. Synthesis and biological evaluation of new benzimidazole-1, 2, 3-triazole hybrids as potential α-glucosidase inhibitors. Bioorg Chem. 2020;95:103482.
Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8:1128–37.
Safavi M, Ashtari A, Khalili F, Mirfazli SS, Saeedi M, Ardestani SK, et al. Novel quinazolin‐4 (3H)‐one linked to 1, 2, 3‐triazoles: synthesis and anticancer activity. Chem Biol Drug Des. 2018;92:1373–81.
Asgari MS, Mohammadi-Khanaposhtani M, Kiani M, Ranjbar PR, Zabihi E, Pourbagher R, et al. Biscoumarin-1, 2, 3-triazole hybrids as novel anti-diabetic agents: design, synthesis, in vitro α-glucosidase inhibition, kinetic, and docking studies. Bioorg Chem. 2019;92:103206.
Imran S, Taha M, Ismail NH, Kashif SM, Rahim F, Jamil W, et al. Synthesis, in vitro and docking studies of new flavone ethers as α‐glucosidase inhibitors. Chem Biol Drug Des. 2016;87:361–73.
Imran S, Taha M, Ismail NH, Kashif SM, Rahim F, Jamil W, et al. Synthesis of novel flavone hydrazones: in-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Eur J Med Chem. 2015;105:156–1570.
Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:D387–92.
Seul, South Corea: Bioinformatics and Molecular Design Research Center. PreADMET program. 2004. http://preadmet.bmdrc.org.
Pandamooz S, Hadipour A, Akhavan‐Niaki H, Pourghasem M, Abedian Z, Ardekani AM, et al. Short exposure to collagenase and coculture with mouse embryonic pancreas improve human dermal fibroblast culture. Biotechnol Appl Biochem. 2012;59:254–61.
Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS ONE. 2010;5:e12029.
Nissink JW, Murray C, Hartshorn M, Verdonk ML, Cole JC, Taylor R. A new test set for validating predictions of protein–ligand interaction. Proteins. 2002;49:457–71.
Chaudhry F, Choudhry S, Huma R, Ashraf M, al-Rashida M, Munir R, et al. Hetarylcoumarins: synthesis and biological evaluation as potent α-glucosidase inhibitors. Bioorg Chem. 2017;73:1–9.
Schottel BL, Chifotides HT, Dunbar KR. Anion-π interactions. Chem Soc Rev. 2008;37:68–83.
Nadeem M, Mumtaz MW, Danish M, Rashid U, Mukhtar H, Anwar F, et al. Calotropis procera: UHPLC-QTOF-MS/MS based profiling of bioactives, antioxidant and anti-diabetic potential of leaf extracts and an insight into molecular docking. J Food Meas Charact. 2019;13:3206–20.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yavari, A., Mohammadi-Khanaposhtani, M., Moradi, S. et al. α-Glucosidase and α-amylase inhibition, molecular modeling and pharmacokinetic studies of new quinazolinone-1,2,3-triazole-acetamide derivatives. Med Chem Res 30, 702–711 (2021). https://doi.org/10.1007/s00044-020-02680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02680-8