Abstract
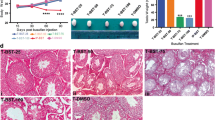
Spermatogonial stem cell transplantation (SSCT) is a strategy that has demonstrated to be feasible to restore spermatogenesis in animal models when it is performed shortly after the gonadotoxic onset to destroy their endogenous germ cells. However, in the case of boys subjected to fertility preservation, future transplantations will be performed with a delay of many years. In order to study how timing of SSCT affects donor-derived spermatogenic recovery in mice, we compared the percentage of spermatogenic tubule cross-sections within testes of 59 C57BL/6NCrl mice distributed in 6 groups: group 1, untreated mice controls (n = 9); group 2, mice that received a single dose of busulfan 40 mg/kg (n = 10); group 3, mice that received two additional doses of busulfan 10 mg/kg every 5 weeks (n = 10); group 4 (SSCT-A), mice subjected to a standard SSCT performed 5 weeks after a single injection of busulfan 40 mg/kg (n = 10); group 5 (SSCT-B), mice subjected to a delayed SSCT performed 15 weeks after a single injection of busulfan 40 mg/kg (n = 10); and group 6 (SSCT-C), mice subjected to a delayed SSCT with two additional doses of busulfan 10 mg/kg every 5 weeks (n = 10). Spermatogenic recovery in standard SSCT-A and SSCT-C groups ranged between 22.29 and 22.65%, compared with a lower recovery rate of 11.54% showed in the SSCT-B group. However, donor contribution resulted higher in standard SSCT-A, representing a 69.71% of cross-sections, compared with the rest of conditions ranging from 34.69 to 35.42%. Overall, we concluded that a delay in the SSCT from the gonadotoxic onset decreases the efficiency of donor-derived spermatogenic recovery in mice.



Similar content being viewed by others
Data availability
All authors declare that all data and materials included in this manuscript comply with field standards.
References
Alves-Lopes JP, Soder O, Stukenborg JB (2017) Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 130:76–89
Baert Y, Van Saen D, Haentjens P, In't Veld P, Tournaye H, Goossens E (2013) What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod 28:1816–1826
Bhang DH, Kim BJ, Kim BG, Schadler K, Baek KH, Kim YH, Hsiao W, Ding BS, Rafii S, Weiss MJ, Chou ST, Kolon TF, Ginsberg JP, Ryu BY, Ryeom S (2018) Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun 9:4379
Brinster RL, Avarbock MR (1994) Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 91:11303–11307
Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saias-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R, Brugnon F, Le Lannou D, Barthelemy C, Rigot JM, Freour T, Berthaut I, Giscard d'Estaing S, Touati F, Melin-Blocquaux MC, Blagosklonov O, Thomas C, Benhamed M, Schmitt F, Kunstmann JM, Thonneau P, Bujan L (2015) Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril 103:478–486 e471
de Michele F, Poels J, Vermeulen M, Ambroise J, Gruson D, Guiot Y, Wyns C (2018) Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front Physiol 9:1413
de Michele F, Poels J, Weerens L, Petit C, Evrard Z, Ambroise J, Gruson D, Wyns C (2017) Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum Reprod 32:32–45
de Rooij DG (2009) The spermatogonial stem cell niche. Microsc Res Tech 72:580–585
Dobrinski I (2006) Germ cell transplantation in pigs--advances and applications. Soc Reprod Fertil Suppl 62:331–339
Dobrinski I, Avarbock MR, Brinster RL (1999) Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod 61:1331–1339
Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, Chu T, Sanfilippo JS, Orwig KE (2013) Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest 123:1833–1843
Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey C, Hanna C, Hennebold JD, Dobrinski I, Orwig KE (2019) Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 363:1314–1319
Gassei K, Valli H, Orwig KE (2014) Whole-mount immunohistochemistry to study spermatogonial stem cells and spermatogenic lineage development in mice, monkeys, and humans. Methods Mol Biol 1210:193–202
Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, Brinster RL, Kolon TF (2010) An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod 25:37–41
Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, Mulhall J, Shnorhavorian M, Brinster RL, Kolon TF (2014) Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer 61:1673–1678
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE (2012) Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 11:715–726
Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S (2002) Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781
Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S (2012) Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res 72:5174–5178
Kadam P, Ntemou E, Baert Y, Van Laere S, Van Saen D, Goossens E (2018) Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res Ther 9:317
Kanatsu-Shinohara M, Morimoto H, Shinohara T (2016) Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol Reprod 94:112
Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O (2007) Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod 22:1384–1395
Kubota H, Brinster RL (2018) Spermatogonial stem cells. Biol Reprod 99:52–74
Martin LA, Assif N, Gilbert M, Wijewarnasuriya D, Seandel M (2014) Enhanced fitness of adult spermatogonial stem cells bearing a paternal age-associated FGFR2 mutation. Stem Cell Reports 3:219–226
Medrano JV, Andres MDM, Garcia S, Herraiz S, Vilanova-Perez T, Goossens E, Pellicer A (2017) Basic and clinical approaches for fertility preservation and restoration in cancer patients. Trends Biotechnol 36:199–215. https://doi.org/10.1016/j.tibtech.2017.10.010
Medrano JV, Martinez-Arroyo AM, Sukhwani M, Noguera I, Quinonero A, Martinez-Jabaloyas JM, Pellicer A, Remohi J, Orwig KE, Simon C (2014) Germ cell transplantation into mouse testes procedure. Fertil Steril 102:e11–e12
Medrano JV, Rombaut C, Simon C, Pellicer A, Goossens E (2016) Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil Steril 106:1539–1549 e1538
Medrano JV, Vilanova-Perez T, Fornes-Ferrer V, Navarro-Gomezlechon A, Martinez-Triguero ML, Garcia S, Gomez-Chacon J, Povo I, Pellicer A, Andres MM, Novella-Maestre E (2018) Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertil Steril 110:1045–1057 e1043
Meistrich ML (2013) Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 100:1180–1186
Ogawa T, Arechaga JM, Avarbock MR, Brinster RL (1997) Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 41:111–122
Onofre J, Faes K, Kadam P, Vicini E, van Pelt AMM, Goossens E (2018) What is the best protocol to cryopreserve immature mouse testicular cell suspensions? Reprod BioMed Online 37:6–17
Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H, van Pelt AM, Eichenlaub-Ritter U, Schlatt S, Diseases ETFOFPIS (2015) A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 30:2463–2475
Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C (2013) Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod 28:578–589
Radford J (2003) Restoration of fertility after treatment for cancer. Horm Res 59(Suppl 1):21–23
Sagaradze G, Basalova N, Kirpatovsky V, Ohobotov D, Nimiritsky P, Grigorieva O, Popov V, Kamalov A, Tkachuk V, Efimenko A (2019) A magic kick for regeneration: role of mesenchymal stromal cell secretome in spermatogonial stem cell niche recovery. Stem Cell Res Ther 10:342
Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, Smith FO, Reaman GH (2010) Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28:2625–2634
Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE (2014) Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril 102:566–580 e567
Wang DZ, Zhou XH, Yuan YL, Zheng XM (2010) Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl 12:263–270
Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, Brichard B (2015) Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod 30:2022–2030
Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, Donnez J (2011) Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod 26:737–747
Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M (2008) Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod 23:2402–2414
Yokonishi T, Sato T, Komeya M, Katagiri K, Kubota Y, Nakabayashi K, Hata K, Inoue K, Ogonuki N, Ogura A, Ogawa T (2014) Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun 5:4320
Acknowledgments
We would like to thank all the staff of the animal facility from the Valencia University for their help and patience. We are also grateful to Dr. Isabel Fariñas from the Valencia University for providing us GFP donors for our experiments.
Funding
This work was supported by a private donation of the Celtic Submarí club- Villareal C.F. to Hospital Universitario y Politécnico La Fe intended to promote the scientific research on fertility preservation in child with cancer and an AES project grant (PI16/00931) conceded by the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Contributions
JVM, ENM, AP, and MMA conceived this work. JVM, IA, ANG, and IN conducted the experiments. JVM analyzed data and wrote the manuscript. All listed authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The use of 71 C57BL/6NCrl 5-wk-old male mice as hosts and 12 Tg(CAG-EGFP)B5Nagy/J 3-wk-old males as donors of testicular cells for this study was approved by the Ethical Committee for Animal Welfare of the University of Valencia (ref.: A1513161658035). Mice were housed in purified air-ventilated racks in order to prevent any infection, fed ad libitum, and with controlled dark/light cycle.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
All statistical analyses were performed using SPSS v.25 (IBM).
Additional information
Editor: Tetsuji Okamoto
Supplementary information
Rights and permissions
About this article
Cite this article
Medrano, J.V., Acimovic, I., Navarro-Gomezlechon, A. et al. Timing of spermatogonial stem cell transplantation affects the spermatogenic recovery outcome in mice. In Vitro Cell.Dev.Biol.-Animal 57, 21–29 (2021). https://doi.org/10.1007/s11626-020-00531-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00531-9