Abstract
During amino acid limitation, the protein kinase Gcn2 phosphorylates the α subunit of eIF2, thereby regulating mRNA translation. In yeast Saccharomyces cerevisiae and mammals, eIF2α phosphorylation regulates translation of related transcription factors Gcn4 and Atf4 through upstream open reading frames (uORFs) to activate transcription genome wide. However, mammals encode three more eIF2α kinases activated by distinct stimuli. Did the translational control system involving eIF2α phosphorylation evolve from so simple (as found in yeast S. cerevisiae) to complex (as found in humans)? Recent genome-wide translational profiling studies of amino acid starvation response in the fission yeast Schizosaccharomyces pombe provide an unexpected answer to this question.



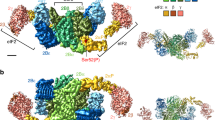
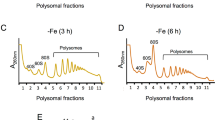
Adapted from Fig. S10B of Chikashige et al. (2020)


adapted from Molecular Biology of the Gene, 7th edition. Right, translational regulatory motifs mediated by uORF-dependent control. In (B), Coherent FFL with AND node makes a persistent detector that only responds to a long-lived signal (Alon, 2007). However, Coherent FFL with OR node works differently, as described in the text
Similar content being viewed by others
References
Alon U (2007) Network motifs: theory and experimental approaches. Nat Rev Genet 8:450–461
Anda S, Zach R, Grallert B (2017) Activation of Gcn2 in response to different stresses. PLoS ONE 12:e0182143
Asano K (2013) Translational Control. In: Dubitzky W, Wolkenhauser O, Cho K-H, Yokota H (eds) Encyclopedia of Systems Biology. Springer, New York, pp 2278–2282
Asano K (2014) Why is start codon selection so precise in eukaryotes? Translation 2:e28387
Asano K, Mizobuchi K (1998) An RNA pseudoknot as the molecular switch for translation of the repZ gene encoding the replication initiator of IncIa plasmid ColIb-P9. J Biol Chem 273:11815–11825
Asano K, Mizobuchi K (2000) Structural analysis of late intermediate complex formed between plasmid ColIb-P9 Inc RNA and its target RNA: how does a single antisense RNA repress translation of two genes at different rates? J Biol Chem 275:1269–1274
Asano K, Moriwaki H, Mizobuchi K (1991) An induced mRNA secondary structure enhances repZ translation in plasmid ColIb-P9. J Biol Chem 266:24549–24556
Bonnet A, Grosso AR, Elkaoutari A, Coleno E, Presle A, Sridhara SC, Janbon G, Géli V, de Almeida SF, Palancade B (2017) Introns protect eukaryotic genomes from transcription-associated genetic instability. Mol Cell 67:608-621.e606
Chen D, Toone WM, Mata J, Lyne R, Burns G, Kivinen K, Brazma A, Jones N, Bähler J (2003) Global transcriptional response of fission yeast to environmental stress. Mol Biol Cell 14:214–229
Chikashige Y, Kato H, Thornton M, Pepper W, Hilgers M, Cecil A, Asano I, Yamada H, Mori C, Brunkow C et al (2020) Gcn2 eIF2α kinase mediates combinatorial translational regulation through nucleotide motifs and uORFs in target mRNAs. Nucleic Acids Res 48:8977–8992
Choy MS, Yusoff P, Lee IC, Newton JC, Goh CW, Page R, Shenolikar S, Peti W (2015) Structural and functional analysis of the GADD34:PP1 eIF2α phosphatase. Cell Rep 11:1885–1891
Dever TE (2002) Gene-specific regulation by general translation factors. Cell 108:545–556
Duncan CDS, Rodríguez-López M, Ruis P, Bähler J, Mata J (2018) General amino acid control in fission yeast is regulated by a nonconserved transcription factor, with functions analogous to Gcn4/Atf4. Proc Natl Acad Sci USA 115:E1829–E1838
Grant CM, Hinnebusch AG (1994) Effect of sequence context at stop codons on efficiency of reinitiation in }U}GCN4}u} translational control. Mol Cell Biol 14:606–618
Hinnebusch AG (1997) Translational regulation of yeast GCN4: a window on factors that control initiator-tRNA binding to the ribosome. J Biol Chem 272:21661–21664
Hinnebusch AG, Dever TE, Asano K (2007) Mechanism of translation initiation in the yeast Saccharomyces cerevisiae. In: Mathews MB, Sonenberg N, Hershey JWB (eds) Translational Control in Biology and Medicine. Cold Spring Harbor Lab Press, Cold Spring Harbor, NY, pp 225–268
Hiraishi H, Oatman J, Haller S, Blunk L, McGivern B, Morris J, Papadopoulos E, Guttierrez W, Gordon M, Bokhari W et al (2014) Essential role of eIF5-mimic protein in animal development is linked to control of ATF4 expression. Nucl Acids Res 42:10321–10330
Hoffman CS, Wood V, Fantes PA (2015) An ancient yeast for young geneticists: a primer on the Schizosaccharomyces pombe model system. Genetics 201:403–423
Hood HM, Neafsey DE, Galagan J, Sachs MS (2009) Evolutionary roles of upstream open reading frames in mediating gene regulation in fungi. Annu Rev Microbiol 63:385–409
Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS (2009) Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324:218–223
Kato H, Kira S, Kawamukai M (2013) The transcription factors Atf1 and Pcr1 are essential for transcriptional induction of the extracellular maltase Agl1 in fission yeast. PLoS ONE 8:e80572
Kearse MG, Wilusz JE (2017) Non-AUG translation: a new start for protein synthesis in eukaryotes. Genes Dev 31:1717–1731
Kozel C, Thompson B, Hustak S, Moore C, Nakashima A, Singh CR, Reid M, Cox C, Papadopoulos E, Luna RE et al (2016) Overexpression of eIF5 or its protein mimic 5MP perturbs eIF2 function and induces ATF4 translation through delayed re-initiation. Nucl Acids Res 44:8704–8713
Lee YY, Cevallos RC, Jan E (2009) An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem 284:6661–6673
Loughran G, Zhdanov AV, Mikhaylova MS, Rozov FN, Datskevich PN, Kovalchuk SI, Serebryakova MV, Kiniry SJ, Michel AM, O’Connor PBF et al (2020) Unusually efficient CUG initiation of an overlapping reading frame in POLG mRNA yields novel protein POLGARF. Proc Natl Acad Sci USA 117:24936–24946
Martín R, Berlanga JJ, de Haro C (2013) New roles of the fission yeast eIF2α kinases Hri1 and Gcn2 in response to nutritional stress. J Cell Sci 126:3010–3020
Mueller PP, Hinnebusch AG (1986) Multiple upstream AUG codons mediate translational control of GCN4. Cell 45:201–207
Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ (2001) Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol 21:4347–4368
Nemoto N, Udagawa T, Ohira T, Jiang L, Hirota K, Wilkinson CRM, Bähler J, Jones N, Ohta K, Wek RC et al (2010) The roles of stress-activated Sty1 and Gcn2 kinases and protooncoprotein homologue Int6/eIF3e in responses to endogenous oxidative stress during histidine starvation. J Mol Biol 404:183–201
Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153:1011–1022
Ogden PJ, Kelsic ED, Sinai S, Church GM (2019) Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 366:1139–1143
Parenteau J, Maignon L, Berthoumieux M, Catala M, Gagnon V, Abou Elela S (2019) Introns are mediators of cell response to starvation. Nature 565:612–617
Piccirillo CA, Bjur E, Topisirovic I, Sonenberg N, Larsson O (2014) Translational control of immune responses: from transcripts to translatomes. Nat Immunol 15:503–511
Riley R, Haridas S, Wolfe KH, Lopes MR, Hittinger CT, Göker M, Salamov AA, Wisecaver JH, Long TM, Calvey CH et al (2016) Comparative genomics of biotechnologically important yeasts. Proc Natl Acad Sci USA 113:9882–9887
Ron D, Harding HP (2007) eIF2alpha phosphorylation in cellular stress responses and disease. In: Mathews MB, Sonenberg N, Hershey JWB (eds) Translational Control in Biology and Medicine. Cold Spring Harbor Lab Press, Cold Spring Harbor, NY, pp 345–386
Sato K, Masuda T, Hu Q, Tobo T, Gillaspie S, Niida A, Thornton M, Kuroda Y, Eguchi H, Nakagawa T et al (2019) Novel oncogene 5MP1 reprograms c-Myc translation initiation to drive malignant phenotypes in colorectal cancer. EBioMed 44:387–402
Singh CR, Watanabe R, Zhou D, Jennings MD, Fukao A, Lee B-J, Ikeda Y, Chiorini JA, Fujiwara T, Pavitt GD et al (2011) Mechanisms of translational regulation by a human eIF5-mimic protein. Nucl Acids Res 39:8314–8328
Steiner WW, Smith GR (2005) Optimizing the nucleotide sequence of a meiotic recombination hotspot in Schizosaccharomyces pombe. Genetics 169:1973–1983
Tang L, Morris J, Wan J, Moore C, Gillaspie S, Aube E, Fujita Y, Nanda J, Anderson A, Cox C et al (2017) Competition between translation initiation factor eIF5 and its mimic protein 5MP determines non-AUG initiation rate genome-wide. Nucl Acids Res 45:11941–11953
Taylor JW, Berbee ML (2006) Dating divergences in the fungal tree of life: review and new analyses. Mycologia 98:838–849
Todd RB, Zhou M, Ohm RA, Leeggangers HA, Visser L, de Vries RP (2014) Prevalence of transcription factors in ascomycete and basidiomycete fungi. BMC Genomics 15:214
Udagawa T, Nemoto N, Wilkinson C, Narashimhan J, Watt S, Jiang L, Zook A, Jones N, Wek RC, Bähler J et al (2008) Int6/eIF3e promotes general translation and Atf1 abundance to modulate Sty1 MAP kinase-dependent stree response in fission yeast. J Biol Chem 283:22063–22075
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 101:11269–11274
Wang DY, Kumar S, Hedges SB (1999) Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc Biol Sci 266:163–171
Wek RC, Staschke KA (2010) How do tumors adapt to nutrient stress? EMBO J 29:1946–1947
Yanofsky C (2000) Transcription attenuation: once viewed as a novel regulatory strategy. J Bacteriol 182:1–8
Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis D, Bobrovnikova-Marjon E, Alan Diehl J, Ron D, Koumenis C (2010) The GCN2-ATF4 pathway is critical for tumor cell survival and proliferation in response to nutrient deprivation. EMBO J 29:2082–2094
Young SK, Wek RC (2016) Upstream open reading frames differentially regulate gene-specific translation in the integrated stress response. J Biol Chem 291:16927–16935
Young SK, Willy JA, Wu C, Sachs MS, Wek RC (2015) Ribosome reinitiation directs gene-specific translation and regulates the integrated stress response. J Biol Chem 290:28257–28271
Zhan K, Vattem KM, Bauer BN, Dever TE, Chen J-J, Wek RC (2002) Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stress. Mol Cell Biol 22:7134–7146
Zhan K, Narasimhan J, Wek RC (2004) Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics 168:1867–1875
Zhou F, Zhang H, Kulkarni SD, Lorsch JR, Hinnebusch AG (2020) eIF1 discriminates against suboptimal initiation sites to prevent excessive uORF translation genome-wide. RNA 26:419–438
Acknowledgements
I thank Hiroaki Kato (School of Medicine, Shimane University), Yuji Chikashige (National Institute of Information and Communications Technology, Kobe), Shintaro Iwasaki (RIKEN, Wako), and Richard Todd (Department of Plant Pathology, Kansas State University) for helpful discussion.
Funding
K-INBRE program Pilot Grant, National Institutes of Health [P20 GM103418]; National Institutes of Health R15 grant [GM125671]; National Science Foundation Research Grant [1412250]; JSPS KAKENHI [18K19963]; Kansas State University (KSU) Terry Johnson Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asano, K. Origin of translational control by eIF2α phosphorylation: insights from genome-wide translational profiling studies in fission yeast. Curr Genet 67, 359–368 (2021). https://doi.org/10.1007/s00294-020-01149-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-020-01149-w