Abstract
Osteoclasts are crucial cellular components of bone and are the cause of various bone problems like osteoporosis. Various biological activities such as anti-tumorous, anti-inflammatory, antibacterial, and immunomodulatory function are influenced by Sclareol, as a natural diterpene compound. However, studies on the effect and mechanism of Sclareol on osteoporosis are rare. In the current research, the influence of Sclareol on osteoclastogenesis and osteoblastogenesis was targeted to be discovered in ovariectomy (OVX)-induced animal models and in vitro. The expression levels of osteoclast-related genes such as c-Fos, NFATc1, and CTSK were detected by RT-qPCR and western blotting to understand the inhibition of Sclareol on the creation of osteoclast. The influence of Sclareol on osteoblastogenesis and the expression of osteoblastogenic markers were also examined. Sclareol inhibited the osteoclastogenesis caused by receptor activator of nuclear factor-κB ligand (RANKL) which promoted osteoblastogenesis through upregulating the expression of cysteine-rich protein 61 (CYR61/CCN1), which is a matricellular protein of the CCN family. The p-ERK and p-P38 protein expression levels were considerably downregulated by Sclareol. Furthermore, CCN1 overexpression partially mimicked the inhibitory effect of Sclareol, while the opposite results were obtained after CCN1 silencing. Additionally, Sclareol protected against loss of bones in an osteoporosis mouse model generated by OVX. The acquired results indicated that Sclareol represses RANKL-induced osteoclastogenesis and promotes osteoblastogenesis via promoting the expression of CCN1 by constraining the mitogen-activated protein kinase (MAPK) pathway. Our findings proposed that for the avoidance and treatment of osteoclast-linked disorders, Sclareol is a potentially effective drug.
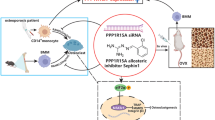
Graphical abstract

A proposed model for mediated regulation of osteoclastogenesis and osteoblastogenesis by Sclareol. The basic model of the process by which Sclareol prevents osteoclastogenesis and promotes osteoblastogenesis. Sclareol may increase the expression of CCN1 through inhibiting the MAPK pathway, thereby inhibiting osteoclast differentiation and attenuating bone resorption. Sclareol represses the expression of c-Fos, which stimulates the formation of osteoclast. In contrast, Sclareol promotes osteoblast differentiation by upregulating Runx2 expression, thereby improving the formation of bones. Consequently, Sclareol protects against loss of bones by regulating the stability of bone makeover via inhibition of bone formation and stimulation of bone resorption.
Graphical Headlights
1. Sclareol represses RANKL-induced osteoclastogenesis.
2. Sclareol promotes osteoblast differentiation.
3. Sclareol inhibits the MAPK pathway through induction of CCN1.
4. Sclareol protects against bone loss by regulating the balance of bone remodeling via inhibition of bone formation and stimulation of bone resorption.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–9.
Bharti A, Aggarwal B. Ranking the role of RANK ligand in apoptosis. Apoptosis. 2004;9:677–90.
Boerckel JD, Mason DE, McDermott AM, Alsberg E. Microcomputed tomography: approaches and applications in bioengineering. Stem Cell Res Ther. 2014;5:144.
Brigstock D. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–75.
Chen CY, Su CM, Hsu CJ, Huang CC, Wang SW, Liu SC, et al. CCN1 promotes VEGF production in osteoblasts and induces endothelial progenitor cell angiogenesis by inhibiting miR-126 expression in rheumatoid arthritis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32:34–45.
Crockett JC, Schutze N, Tosh D, Jatzke S, Duthie A, Jakob F, et al. The matricellular protein CYR61 inhibits osteoclastogenesis by a mechanism independent of alphavbeta3 and alphavbeta5. Endocrinology. 2007;148(12):5761–8. https://doi.org/10.1210/en.2007-0473.
David J-P, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and-independent mechanisms. J Cell Sci. 2002;115:4317–25.
Dimas K, Hatziantoniou S, Tseleni S, Khan H, Georgopoulos A, Alevizopoulos K, et al. Sclareol induces apoptosis in human HCT116 colon cancer cells in vitro and suppression of HCT116 tumor growth in immunodeficient mice. Apoptosis. 2007;12:685–94.
Duan G, Hou S, Ji J, Deng B. The study of sclareol in inhibiting proliferation of osteosarcoma cells by apoptotic induction and loss of mitochondrial membrane potential. Cancer biomarkers : section A of Disease markers. 2018;22:29–34.
Frey SP, Doht S, Eden L, Dannigkeit S, Schuetze N, Meffert RH, et al. Cysteine-rich matricellular protein improves callus regenerate in a rabbit trauma model. Int Orthop. 2012;36:2387–93.
Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis research & therapy. 2007;9:103.
Han G, Zuo J, Holliday LSJB. Specialized roles for actin in osteoclasts: unanswered questions and therapeutic opportunities. Biomolecules. 2019;9:17.
Hatziantoniou S, Dimas K, Georgopoulos A, Sotiriadou N, Demetzos C. Cytotoxic and antitumor activity of liposome-incorporated sclareol against cancer cell lines and human colon cancer xenografts. Pharmacol Res. 2006;53:80–7.
Hsieh Y-H, Deng J-S, Pan H-P, Liao J-C, Huang S-S, Huang G-J. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int Immunopharmacol. 2017;44:16–25.
Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068–74.
Huang G-J, Pan C-H, Wu C-H. Sclareol exhibits anti-inflammatory activity in both lipopolysaccharide-stimulated macrophages and the λ-carrageenan-induced paw edema model. J Nat Prod. 2012;75:54–9.
Jahan K, Manickam G, Tabrizian M, Murshed M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci Rep. 2020;10:1–17.
James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica. 2013,(2013-12-12);2013(2013):684736.
Jin H, Shao Z, Wang Q, Miao J, Bai X, Liu Q, et al. Sclareol prevents ovariectomy-induced bone loss in vivo and inhibits osteoclastogenesis in vitro via suppressing NF-kappaB and MAPK/ERK signaling pathways. Food Funct. 2019;10:6556–67.
Johnson SK, Stewart JP, Bam R, Qu P, Barlogie B, van Rhee F, et al. CYR61/CCN1 overexpression in the myeloma microenvironment is associated with superior survival and reduced bone disease. Blood. 2014;124:2051–60.
Kaushik R, Narayanan P, Vasudevan V, Muthukumaran G, Usha A. Nutrient composition of cultivated stevia leaves and the influence of polyphenols and plant pigments on sensory and antioxidant properties of leaf extracts. J Food Sci Technol. 2010;47:27–33.
Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005;280:32905–13.
Kong Y-Y, Yoshida H, Sarosi I, Tan H-L, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23.
Lau LF. Cell surface receptors for CCN proteins. Journal of cell communication and signaling. 2016;10:121–7.
Lee NK. Molecular understanding of osteoclast differentiation and physiology. Endocrinol Metab. 2010;25:264–9.
Lee S, Shin S, Jung E, Park D. Cell-based assay system for high-throughput screening of anti-photo-aging agents in fibroblast transfectants. Cytotechnology. 2016;68:1633–40.
Li C, Yang Z, Li Z, Ma Y, Zhang L, Zheng C, et al. Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating RANKL-mediated NF-κB and MAPK signaling pathways. J Bone Miner Res. 2011;26:644–56.
Liu H, Peng F, Liu Z, Jiang F, Li L, Gao S, et al. CYR61/CCN1 stimulates proliferation and differentiation of osteoblasts in vitro and contributes to bone remodeling in vivo in myeloma bone disease. Int J Oncol. 2017;50:631–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif). 2001;25:402–8.
Lobel M, Bauer S, Meisel C, Eisenreich A, Kudernatsch R, Tank J, et al. CCN1: a novel inflammation-regulated biphasic immune cell migration modulator. Cellular and molecular life sciences : CMLS. 2012;69:3101–13.
Miyazaki T, Tokimura F, Tanaka S. A review of denosumab for the treatment of osteoporosis. Patient Preference & Adherence. 2014;8:463–71.
Monje P, Hernández-Losa J, Lyons RJ, Castellone MD, Gutkind JS. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem. 2005;280:35081–4.
Noori S, Mohammad Hassan Z, Salehian O. Sclareol reduces CD4+ CD25+ FoxP3+ T-reg cells in a breast cancer model in vivo. Iran J Immunol. 2013;10:10–21.
Roscher A, Hasegawa T, Dohnke S, Ocana-Morgner C, Amizuka N, Jessberger R, et al. The F-actin modulator SWAP-70 controls podosome patterning in osteoclasts. Bone Rep. 2016;5:214–21.
Schett G. Cells of the synovium in rheumatoid arthritis. Osteoclasts Arthritis research & therapy. 2007;9:203.
Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–64.
Soltanoff C, Yang S, Li YW. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene Exp. 2009;19:1.
Su JL, Chiou J, Tang CH, Zhao M, Tsai CH, Chen PS, et al. CYR61 regulates BMP-2-dependent osteoblast differentiation through the {alpha}v{beta}3 integrin/integrin-linked kinase/ERK pathway. J Biol Chem. 2010;285:31325–36.
Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature. 2002;416:744–9.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8.
Toshio K, Naohisa W, Akiko K, Takashi K, Ferry S, Kazuko T, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200:941–6.
Tsai S-W, Hsieh M-C, Li S, Lin S-C, Wang S-P, Lehman C, et al. Therapeutic potential of sclareol in experimental models of rheumatoid arthritis. Int J Mol Sci. 2018;19:1351.
Tyagi AK, Prasad S, Majeed M, Aggarwal BB. Calebin A downregulates osteoclastogenesis through suppression of RANKL signalling. Arch Biochem Biophys. 2016;593:80–9.
Wei Z-F, Tong B, Xia Y-F, Lu Q, Chou G-X, Wang Z-T, et al. Norisoboldine suppresses osteoclast differentiation through preventing the accumulation of TRAF6-TAK1 complexes and activation of MAPKs/NF-κB/c-Fos/NFATc1 pathways. PloS one. 2013;8:e59171.
Zhang Q, Wu J, Cao Q, Xiao L, Wang L, He D, et al. A critical role of Cyr61 in interleukin-17-dependent proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2009;60:3602–12.
Zhang LM, Su PQ, Xu CX, Chen CH, Liang AJ, Du KL, et al. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J Pineal Res. 2010;49:364–72.
Zhang Y, Sheu TJ, Hoak D, Shen J, Hilton MJ, Zuscik MJ, et al. CCN1 regulates chondrocyte maturation and cartilage development. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31:549–59.
Zhao G, Huang BL, Rigueur D, Wang W, Bhoot C, Charles KR, et al. CYR61/CCN1 regulates sclerostin levels and bone maintenance. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018;33:1076–89.
Zhao G, Kim EW, Jiang J, Bhoot C, Charles KR, Baek J, et al. CCN1/Cyr61 is required in osteoblasts for responsiveness to the anabolic activity of PTH. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2020;35:2289–300.
Funding
This work was supported by A Project of Scientific Research Fund of the Zhejiang Provincial Education Department (188310-542126/015). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Contributions
Fengchao Zhao and Xiang Li contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Xiang Li and Yuxin Wang. The first draft of the manuscript was written by Xiang Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All animal studies were carried out in conformity to the National Institutes of Health (NIH) guidelines and protocols for laboratory animal use and care. The study was approved by the Animal Experimental Ethical Committee of the First Affiliated Hospital College of Medicine, Zhejiang University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1.
Sclareol promotes BMSCs differentiation into OB and the expression of the osteoblast-specific genes via modulating CCN1 expression. (A) Effects of Sclareol on the viability of BMSCs by CCK8 assays at 48h and 96 h. (B) ALP and ARS expression in BMSCs after their treatment with various concentrations of Sclareol for seven days. Alizarin Red S staining for 21 days was used to determine the mineralized extracellular matrix. (C) The ALP-positive cells number and the OD values were obtained for mineralized matrix solutions after their treatment with Sclareol. (D) Expression levels of the CCN1, OPN, OCN, and Runx2 in BMSCs treated-with Sclareol were detected using RT-qPCR. (E) The expression levels of osteoblast-specific genes (Runx2) and CCN1 in BMSCs treated with indicated concentrations of Sclareol. (F) The CCN1 expression in BMSCs after the treatment with Sclareol. (G) Immunofluorescence was used to detect CCN1 expression in BMSCs. All experiments were carried out in triplicate. The results were presented as mean ± SEM. *P < 0.05 and **P < 0.01 in comparison to control group. (PNG 79122 kb)
Supplementary Figure 2.
CCN1 silencing promotes osteoblastogenesis and the expression of osteoblast-specific gene expression in BMSCs. (A) The transfection efficiency of the CCN1 vector and siCCN1 were detected by western blot. (B) The CCN1 expression in BMSCs following transfection with CCN1 siRNA or CCN1 vector and Sclareol treatment. ALP-positive cells number and OD values following transfection with CCN1 siRNA (C) and CCN1 vector (D). The untreated cells served as a control in this study. (E-G) The expression of osteoblast-specific genes in BMSCs after the transfection with CCN1 siRNA or CCN1 vector was detected using western blot and RT-qPCR. (H) Treatment of BMSCs with or without 0.5 μM of Sclareol for the designated times. The protein expression levels of AKT, p-AKT, p-P38, and P38 were evaluated. (I) The effects of MAPK pathway inhibitor, SB203580, on the protein expression of CCN1 induced by Sclareol in BMSCs. (J, K) The effect of MAPK pathway inhibitor, SB203580, on the ALP and ARS staining induced by Sclareol in BMSCs. All experiments were carried out in triplicate. The results were presented as mean ± SEM. *P < 0.05 and **P < 0.01 in comparison to control group. (PNG 3147 kb)
Rights and permissions
About this article
Cite this article
Li, X., Wang, Y., Li, L. et al. Sclareol inhibits RANKL-induced osteoclastogenesis and promotes osteoblastogenesis through promoting CCN1 expression via repressing the MAPK pathway. Cell Biol Toxicol 37, 849–871 (2021). https://doi.org/10.1007/s10565-020-09578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09578-6