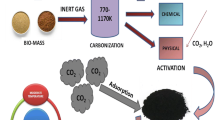
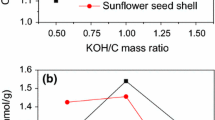
Abstract
Increasing ambient carbon dioxide (CO2) concentration from anthropogenic greenhouse gas emission has contributed to the growing rate of global land and ocean surface temperature. Various carbon capture and storage (CCS) technologies were established to mitigate this impending issue. CO2 adsorption is gaining prominence since unlike traditional chemical absorption, it does not require high energy usage for solvent regeneration and consumption of corrosive chemical solvent. In CO2 adsorption, activated carbons show high CO2 adsorption capacity given their well-developed porous structures. Numerous researches employed oil palm wastes as low-cost precursors. This paper provides a comprehensive assessment of research works available thus far in oil palm-derived activated carbon (OPdAC) for CO2 adsorption application. First, we present the desired OPdAC characteristics and its precursors in terms of their chemical properties, elemental, and proximate compositions. This is followed by an overview of various activation methodologies and surface modification methods to attain the desired characteristics for CO2 adsorption. Then the focus turned to present available OPdAC CO2 adsorption performance and how it is affected by its physical and chemical characteristics. Based on these, we identify the challenges and the potential development in different aspects such as precursor selection, process development, and optimization of parameter. A pilot scale production cost analysis is also presented to compare various activation and surface modification methods, so that the appropriate method can be selected for CO2 adsorption.














Similar content being viewed by others
Change history
15 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42823-021-00232-7
Abbreviations
- OPdAC:
-
Oil palm-derived activated carbon
- CO2 :
-
Carbon dioxide
- CCS:
-
Carbon capture and storage
- DAC:
-
Direct air capture
- CH4 :
-
Methane
- NH2COOH:
-
Carbamic acid
- MO:
-
Metal oxide
- NOx :
-
Nitrogen oxides
- SOx :
-
Sulfur oxides
- BET:
-
Brunauer–Emmett–Teller
- MF:
-
Mesocarp fibers
- PKS:
-
Palm kernel shells
- EFB:
-
Empty fruit bunches
- POME:
-
Palm oil mill effluent
- OPT:
-
Oil palm trunks
- OPL:
-
Oil palm leaves
- OPF:
-
Oil palm frond
- POS:
-
Palm oil sludge
- CHNS-O:
-
Carbon, hydrogen, nitrogen, sulfur, and oxygen
- N2 :
-
Nitrogen
- CO:
-
Carbon monoxide
- H2 :
-
Hydrogen
- SEM:
-
Scanning electron micrograph
- KOH:
-
Potassium hydroxide
- NaOH:
-
Sodium hydroxide
- K2CO3 :
-
Potassium carbonate
- H3PO4 :
-
Phosphoric acid
- H2SO4 :
-
Sulfuric acid
- ZnCl2 :
-
Zinc chloride
- K2O:
-
Potassium oxide
- H2O:
-
Water
- K:
-
Metallic potassium
- Na2CO3 :
-
Sodium carbonate
- PSAC:
-
Palm shell porous carbon
- PAC:
-
Physicochemically porous activated carbon
- NH3 :
-
Ammonia
- PEI:
-
Polyethyleneimine
- AMPD:
-
2-Amino-2-methyl-1,3-propanediol
- AMP:
-
2-Amino-2-methyl-1-propanol
- MMEA:
-
2-(Methylamino)ethanol
- MEA:
-
Mono-ethanolamine
- DEA:
-
Diethanolamine
- EDA:
-
Ethylenediamine
- DETA:
-
Diethylenetriamine
- NH2 :
-
Amino radical
- NH:
-
Imidogen radical
- HCN:
-
Hydrogen cyanide
- (CN)2 :
-
Cyanogen
- -CN:
-
Cyano radical
- GAC:
-
Granular palm kernel shell-based activated carbon
- MDEA:
-
Methyl diethanolamine
- TEPA:
-
Tetra ethylene pentamine
- PEHA:
-
Penta ethylene-hexamine
- Mg:
-
Magnesium
- Ca:
-
Calcium
- Cu:
-
Copper
- Co:
-
Cobalt
- Ni:
-
Nickel
- Fe:
-
Iron
- Cr:
-
Chromium
- HNO3 :
-
Nitric acid
- Cu2 + :
-
Cupric ion
- Zn2 + :
-
Zinc ion
- Ni(NO3)2·6H2O:
-
Nickel nitrate hexahydrate
- NH4VO3 :
-
Ammonium metavanadate
- Ce(NO3)3·6H2O:
-
Cerium nitrate
- Fe(NO3)3·9H2O:
-
Ferum nitrate
- MgO:
-
Magnesium oxide
- CuO:
-
Copper oxide
- CeO2 :
-
Cerium oxide
- TiO2 :
-
Titanium oxide
- BaO:
-
Barium oxide
- H2S:
-
Hydrogen sulphide
- TSA:
-
Temperature swing adsorption
- VSA:
-
Vacuum swing adsorption
- VTSA:
-
Vacuum and temperature swing adsorption
References
National Oceanic and Atmospheric Administration (NOAA) National Centers for Environmental Information (2019) Climate at a glance. https://www.ncdc.noaa.gov/cag/global/time-series/globe/land_ocean/ytd/12/1880-2016. Accessed 7 Oct 2019
National Oceanic and Atmospheric Administration (NOAA) National Centers for Environmental Information (2020) State of the climate: global climate report for January 2020. https://www.ncdc.noaa.gov/sotc/global/202001. Accessed 20 Feb 2020
Etheridge DM, Steele LP, Langenfelds RL, Francey RJ (2001) Law dome atmospheric CO2 data (1800–1850). https://www1.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/law/law_co2.txt. Accessed 7 Oct 2019
National Oceanic and Atmospheric Administration (NOAA) Earth System Research Laboratory for Global Monitoring Division (2019) Recent monthly average Mauna Loa CO2. https://www.esrl.noaa.gov/gmd/ccgg/trends/. Accessed 7 Oct 2019
National Oceanic and Atmospheric Administration (NOAA) Earth System Research Laboratory for Global Monitoring Division (2020) Recent monthly average Mauna Loa CO2. https://www.esrl.noaa.gov/gmd/ccgg/trends/mlo.html. Accessed 20 Feb 2020
Oh TH (2010) Carbon capture and storage potential in coal-fired plant in Malaysia—a review. Renew Sustain Energy Rev 14:2697–2709
Zhao HY, Luo XN, Zhang HJ, Sun NN, Wei W, Sun YH (2018) Carbon-based adsorbents for post-combustion capture: a review. Greenh Gases Sci Technol 8:11–36
Spigarelli BP, Kawatra SK (2013) Opportunities and challenges in carbon dioxide capture. J CO2 Util 1:69–87
Rashidi NA, Yusup S (2016) An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J CO2 Util 13:1–16
Yoro KO, Sekoai PT (2016) The potential of CO2 capture and storage technology in south Africa’s coal-fired thermal power plants. Environments 3:1–20
Socolow R, Desmond M, Aines R, Blackstock J, Bolland O, Kaarsberg T, Lewis N, Mazzotti M, Pfeffer A, Sawyer K, Siirola J, Smit B, Wilcox J (2011) Direct air capture of CO2 with chemicals. A Technology Assessment for the APS Panel on Public Affairs
Modak A, Jana S (2019) Advancement in porous adsorbents for post-combustion CO2 capture. Micropor Mesopor Mater 276:107–132
Veselovskaya JV, Derevschikov VS, Kardash TY, Stonkus OA, Trubitsina TA, Okunev AG (2013) Direct CO2 capture from ambient air using K2CO3/Al2O3 composite sorbent. Int J Greenh Gas Con 17:332–340
Theo WL, Lim JS, Hashim H, Mustaffa AA, Ho WS (2016) Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl Energy 183:1633–1663
Jansen D, Gazzani M, Manzolini G, Ev D, Carbo M (2015) Pre-combustion CO2 capture. Int J Greenh Gas Con 40:167–187
Sanz-Pérez ES, Murdock CR, Didas SA, Jones CW (2016) Direct capture of CO2 from ambient air. Chem Rev 116:11840–11876
Hornbostel MD, Bao JE, Krishnan G, Nagar A, Jayaweera I, Kobayashi T, Sanjurjo A, Sweeney J, Carruthers D, Petruska MA, Dubois L (2013) Characteristics of an advanced carbon sorbent for CO2 capture. Carbon 56:77–85
CO2 capture technologies: technology options for CO2 capture (2012) http://hub.globalccsinstitute.com/sites/default/files/publications/29701/co2-capture-technologies.pdf. Accessed 16 Oct 2018
Ho MT, Allinson GW, Wiley DE (2008) Reducing the cost of CO2 capture from flue gases using pressure swing adsorption. Ind Eng Chem Res 47:4883–4890
Yu CH, Huang CH, Tan CS (2012) A review of CO2 capture by absorption and adsorption. Aerosol Air Qual Res 12:745–769
Ben-Mansour R, Habib MA, Bamidele OE, Basha M, Qasem NAA, Peedikakkal A, Laoui T, Ali M (2016) Carbon capture by physical adsorption: materials, experimental investigations and numerical modeling and simulations—a review. Appl Energy 161:225–255
Chue KT, Kim JN, Yoo YJ, Cho SH, Yang RT (1995) Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind Eng Chem Res 34:591–598
Xiao P, Zhang J, Webley P, Li G, Singh R, Todd R (2008) Capture of CO2 from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption 14:575–582
Plaza MG, García S, Rubiera F, Pis JJ, Pevida C (2010) Post-combustion CO2 capture with a commercial activated carbon: comparison of different regeneration strategies. Chem Eng J 163:41–47
Chowdhury S, Balasubramanian R (2016) Highly efficient, rapid and selective CO2 capture by thermally treated graphene nanosheets. J CO2 Util 13:50–60
Dinda S (2013) Development of solid adsorbent for carbon dioxide capture from flue gas. Sep Purif Technol 109:64–71
Goto K, Kazama S, Furukawa A, Serizawa M, Aramaki S, Shoji K (2013) Effect of CO2 purity on energy requirement of CO2 capture processes. Energy Proced 37:806–812
Wilcox J, Haghpanah R, Rupp EC, He JJ, Lee KJ (2014) Advancing adsorption and membrane-separation processes for the gigaton carbon capture challenge. Annu Rev Chem Biomol 5:479–505
Kiener J, Limousy L, Jeguirim M, Le Meins J-M, Hajjar-Garreau S, Bigoin G, Ghimbeu MC (2019) Activated carbon/transition metal (Ni, In, Cu) hexacyanoferrate nanocomposites for cesium adsorption. Mater 12:1–17
Rehman A, Park M, Park SJ (2019) Current progress on the surface chemical modification of carbonaceous materials. Coatings 9:1–21
Samer M (2015) Biological and chemical wastewater treatment processes. Wastewater Treat Eng:1–50
Fellinger T (2017) Sol–gel carbons from ionothermal syntheses. J Sol-Gel Sci Technol 81:52–58
Tsuchiya T (2018) Van der waals force. In: White WM, Casey WH, Marty B, Yurimoto H (eds) Encyclopedia of geochemistry. Springer, Cham
Coriani S, Halkier A, Rizzo A, Ruud K (2000) On the molecular electric quadrupole moment and the electric-field-gradient-induced birefringence of CO2 and CS2. Chem Phys Lett 326:269–276
Bae YS, Farha OK, Spokoyny AM, Mirkin CA, Hupp JT, Snurr RQ (2008) Carborane-based metal–organic frameworks as highly selective sorbents for CO2 over methane. Chem Commun 4135–4137
Chowdhury P, Mekala S, Dreisbach F, Gumma S (2012) Adsorption of CO, CO2 and CH4 on Cu-BTC and MIL-101 metal organic frameworks: effect of open metal sites and adsorbate polarity. Micropor Mesopor Mater 152:246–252
Freeman B, Yampolskii Y, Pinnau I (2006) Materials science of membranes for gas and vapor separation. Wiley, New York
Martín-Martínez JM, Torregrosa-Maciá R, Mittelmeijer-Hazeleger MC (1995) Mechanisms of adsorption of CO2 in the micropores of activated anthracite. Fuel 74:111–114
Singh G, Kim IY, Lakhi KS, Srivastava P, Naidu R, Vinu A (2017) Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon 116:448–455
Nasri N, Hamza U, Amin Noraishah S, Mohammed J, Ahmed M, Zain H, Bahru J (2014) Kinetics of CO2 adsorption on microwave palm shell activated carbon. Adv Mater Res 1043:224–228
Hamza UD, Nasrib NS, Amina NS, Zainc HM, Mohammedd J (2015) CO2 adsorption equilibria on a hybrid palm shell-PEEK porous carbons. Chem Eng Trans 45:301–306
Parshetti GK, Chowdhury S, Balasubramanian R (2015) Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 148:246–254
Rashidi NA, Yusup S, Borhan A, Loong LH (2014) Experimental and modelling studies of carbon dioxide adsorption by porous biomass derived activated carbon. Clean Technol Environ 16:1353–1361
Lee SP, Mellon N, Shariff AM, Leveque JM (2018) High-pressure CO2–CH4 selective adsorption on covalent organic polymer. J Nat Gas Sci Eng 50:139–146
Jenkins HDB (2008) Chemical thermodynamics at a glance. Blackwell Publishing Ltd, Oxford, pp 1–195
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology, 4th edn. W. H Freeman, New York
Houshmand A, Daud WMAW, Lee MG, Shafeeyan MS (2012) Carbon dioxide capture with amine-grafted activated carbon. Water Air Soil Pollut 223:827–835
Lee CS, Ong YL, Aroua MK, Daud WMAW (2013) Impregnation of palm shell-based activated carbon with sterically hindered amines for CO2 adsorption. Chem Eng J 219:558–564
Khalil SH, Aroua MK, Daud WMAW (2012) Study on the improvement of the capacity of amine-impregnated commercial activated carbon beds for CO2 adsorbing. Chem Eng J 183:15–20
Hidayu AR, Muda N (2016) Preparation and characterization of impregnated activated carbon from palm kernel shell and coconut shell for CO2 capture. Proced Eng 148:106–113
Hidayu ARN, Muda N (2017) Impregnated palm kernel shell activated carbon for CO2 adsorption by pressure swing adsorption. Indian J Sci Technol 10:1–6
Skoog DA, West DM, Holler FJ, Crouch SR (2013) Fundamentals of analytical chemistry, 9th edn. Cengage Learning, Belmont
Lide DR (1995) CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data, 84th edn. CRC Press, Boca Raton
Kim BJ, Cho KS, Park SJ (2010) Copper oxide-decorated porous carbons for carbon dioxide adsorption behaviors. J Colloid Interface Sci 342:575–578
Aziz MAA, Jalila AA, Triwahyonoc S, Ahmada A (2015) CO2 methanation over heterogeneous catalysts: recent progress and future prospects. Green Chem 17:2647–2663
Zumdahl SS, DeCoste DJ (2016) Chemical principles, 8th edn. Cengage Learning, Boston
Isahak WNRW, Ramli ZAC, Ismail MW, Ismail K, Yusop RM, Hisham MWM, Yarmo MA (2013) Adsorption–desorption of CO2 on different type of copper oxides surfaces: physical and chemical attractions studies. J CO2 Util 2:8–15
Bollini P, Didas SA, Jones CW (2011) Amine-oxide hybrid materials for acid gas separations. J Mater Chem 21:15100–15120
Calvo-Muñoz EM, García-Mateos FJ, Rosas JM, Rodríguez-Mirasol J, Cordero T (2016) Biomass waste carbon materials as adsorbents for CO2 capture under post-combustion conditions. Front Mater 3:1–14
Choi SH, Drese JH, Jones CW (2009) Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chemsuschem 2:796–854
Mukherjee A, Okolie JA, Abdelrasoul A, Niu C, Dalai AK (2019) Review of post-combustion carbon dioxide capture technologies using activated carbon. J Environ Sci 83:46–63
Sayari A, Belmabkhout Y, Serna-Guerrero R (2011) Flue gas treatment via CO2 adsorption. Chem Eng J 171:760–774
Rafatullah M, Ahmad T, Ghazali A, Sulaiman O, Danish M, Hashim R (2013) Oil palm biomass as a precursor of activated carbons: a review. Crit Rev Env Sci Technol 43:1117–1161
Zhou YF, Haynes RJ (2010) Sorption of heavy metals by inorganic and organic components of solid wastes: significance to use of wastes as low-cost adsorbents and immobilizing agents. Crit Rev Env Sci Technol 40:909–977
Savova D, Apak E, Ekinci E, Yardim F, Petrov N, Budinova T, Razvigorova M, Minkova V (2001) Biomass conversion to carbon adsorbents and gas. Biomass Bioenergy 21:133–142
Manocha SM (2003) Porous carbons. Sadhana 28:335–348
Dodevski V, Janković B, Stojmenović M, Krstić S, Popović J, Pagnacco MC, Popović M, Pašalić S (2017) Plane tree seed biomass used for preparation of activated carbons (AC) derived from pyrolysis. Modeling the activation process. Colloids Surf, A 522:83–96
Ooi CH, Lee T, Pung SY, Yeoh FY (2013) Activated carbon fiber derived from single step carbonization-activation process. ASEAN Eng J B 4:40–50
Adinata D, Daud WMAW, Aroua MK (2007) Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour Technol 98:145–149
Nasri NS, Hamza UD, Ismail SN, Ahmed MM, Mohsin R (2014) Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J Clean Prod 71:148–157
Rashidi NA, Yusup S (2017a) Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption. J Clean Prod 168:474–486
Kumar A, Jena HM (2016) Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys 6:651–658
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sust Energy Rev 82:1393–1414
Guo SH, Peng JH, Li W, Yang KB, Zhang LB, Zhang SM, Xia HY (2009) Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl Surf Sci 255:8443–8449
Danish M, Ahmad T (2018) A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew Sustain Energy Rev 87:1–21
González AS, Plaza MG, Rubiera F, Pevida C (2013) Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem Eng J 230:456–465
Joseph C, Quek K, Daud W, Moh P (2017) Physical activation of oil palm empty fruit bunch via CO2 activation gas for CO2 adsorption. IOP Conf Ser Mater Sci Eng 206:1–11
Nasri N, Zain H, Usman H, Zulkifli A, Zalilah S, Nurul AS, Nur LA (2015) CO2 adsorption-breakthrough study on activated carbon derived from renewable oil palm empty fruit bunch. Aust J Basic Appl Sci 9:67–71
Sethupathi S, Bashir MJK, Akbar ZA, Mohamed AR (2015) Biomass-based palm shell activated carbon and palm shell carbon molecular sieve as gas separation adsorbents. Waste Manag Res 33:303–312
Lee SY, Park SJ (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11
Rashidi NA, Yusup S (2017b) A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem Eng J 314:277–290
Shafeeyan MS, Daud WMAW, Shamiri A, Aghamohammadi N (2015) Adsorption equilibrium of carbon dioxide on ammonia-modified activated carbon. Chem Eng Res Des 104:42–52
Younas M, Leong LK, Mohamed AR, Sethupathi S (2016) CO2 adsorption by modified palm shell activated carbon (PSAC) via chemical and physical activation and metal impregnation. Chem Eng Commun 203:1455–1463
Aroua M, Daud W, Yin C, Adinata D (2008) Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep Purif Technol 62:609–613
Kongnoo A, Intharapat P, Worathanakul P, Phalakornkule C (2016) Diethanolamine impregnated palm shell activated carbon for CO2 adsorption at elevated temperatures. J Environ Chem Eng 4:73–81
Shafeeyan MS, Daud WMAW, Houshmand A, Arami-Niya A (2011) Ammonia modification of activated carbon to enhance carbon dioxide adsorption: effect of pre-oxidation. Appl Surf Sci 257:3936–3942
Hoseinzadeh Hesas R, Arami-Niya A, Wan Daud WMA, Sahu J (2015) Microwave-assisted production of activated carbons from oil palm shell in the presence of CO2 or N2 for CO2 adsorption. J Ind Eng Chem 24:196–205
Hoseinzadeh Hesas R, Arami-Niya A, Wan Daud WMA, Sahu JN (2013) Comparison of oil palm shell-based activated carbons produced by microwave and conventional heating methods using zinc chloride activation. J Anal Appl Pyrolysis 104:176–184
Yacob AR, Wahab N, Suhaimi NH, Mustajab MKAA (2013) Microwave induced carbon from waste palm kernel shell activated by phosphoric acid. Int J Eng Technol 5:214–217
Foo KY, Hameed BH (2011a) Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue. Desalination 275:302–305
Foo KY, Hameed BH (2011b) Microwave-assisted preparation of oil palm fiber activated carbon for methylene blue adsorption. Chem Eng J 166:792–795
Index Mundi (2017) Palm oil production by country. http://www.indexmundi.com/agriculture/?commodity=palm-oil&graph=production. Accessed 22 Nov 2018
Board MPO (2019) Production of crude palm oil for the month of August 2019. Malaysia
Lee XJ, Lee LY, Gan S, Thangalazhy-Gopakumar S, Ng HK (2017) Biochar potential evaluation of palm oil wastes through slow pyrolysis: thermochemical characterization and pyrolytic kinetic studies. Bioresour Technol 236:155–163
Awalludin MF, Sulaiman O, Hashim R, Nadhari WNAW (2015) An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew Sustain Energy Rev 50:1469–1484
Loh SK (2017) The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers Manag 141:285–298
Omar R, Idris A, Yunus R, Khalid K, Aida Isma MI (2011) Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 90:1536–1544
Yusoff S (2006) Renewable energy from palm oil—innovation on effective utilization of waste. J Clean Prod 14:87–93
Wu XX, Zhang CY, Tian ZW, Cai JJ (2018) Large-surface-area carbons derived from lotus stem waste for efficient CO2 capture. New Carbon Mater 33:252–261
Yang GZ, Song S, Li J, Tang ZH, Ye JY, Yang JH (2018) Preparation and CO2 adsorption properties of porous carbon by hydrothermal carbonization of tree leaves. J Mater Sci Technol 35:875–884
Choi SW, Tang JL, Pol VG, Lee KB (2019) Pollen-derived porous carbon by KOH activation: effect of physicochemical structure on CO2 adsorption. J CO2 Util 29:146–155
Pevida C, Plaza MG, Arias B, Fermoso J, Rubiera F, Pis JJ (2008) Surface modification of activated carbons for CO2 capture. Appl Surf Sci 254:7165–7172
Zhang ZJ, Xu MY, Wang HH, Li Z (2010) Enhancement of CO2 adsorption on high surface area activated carbon modified by N2, H2 and ammonia. Chem Eng J 160:571–577
David E, Kopac J (2014) Activated carbons derived from residual biomass pyrolysis and their CO2 adsorption capacity. J Anal Appl Pyrolysis 110:322–332
Ogungbenro AE, Quang DV, Al-Ali KA, Vega LF, Abu-Zahra MRM (2018) Physical synthesis and characterization of activated carbon from date seeds for CO2 capture. J Environ Chem Eng 6:4245–4252
Ilomuanya MO, Nashiru B, Ifudu ND, Igwilo CI (2017) Effect of pore size and morphology of activated charcoal prepared from midribs of Elaeis guineensis on adsorption of poisons using metronidazole and Escherichia coli O157:H7 as a case study. J Microsc Ultrastruct 5:32–38
Kennedy LJ, Vijaya JJ, Sekaran G (2004) Effect of two-stage process on the preparation and characterization of porous carbon composite from rice husk by phosphoric acid activation. Ind Eng Chem Res 43:1832–1838
Oh GH, Park CR (2002) Preparation and characteristics of rice-straw-based porous carbons with high adsorption capacity. Fuel 81:327–336
Daud WMAW, Ali WSW (2004) Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour Technol 93:63–69
Aworn A, Thiravetyan P, Nakbanpote W (2008) Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J Anal Appl Pyrolysis 82:279–285
Bachrun S, AyuRizka N, Annisa S, Hidayat A (2016) Preparation and characterization of activated carbon from sugarcane bagasse by physical activation with CO2 gas. IOP Conf Ser Mater Sci Eng 105:1–8
Sohni S, Norulaini NAN, Hashim R, Khan SB, Fadhullah W, Mohd Omar AK (2018) Physicochemical characterization of Malaysian crop and agro-industrial biomass residues as renewable energy resources. Ind Crop Prod 111:642–650
Kabir G, Mohd Din AT, Hameed BH (2017) Pyrolysis of oil palm mesocarp fiber and palm frond in a slow-heating fixed-bed reactor: a comparative study. Bioresour Technol 241:563–572
Okahisa Y, Furukawa Y, Ishimoto K, Narita C, Intharapichai K, Ohara H (2018) Comparison of cellulose nanofiber properties produced from different parts of the oil palm tree. Carbohydr Polym 198:313–319
Tiong YW, Yap CL, Gan SY, Yap WSP (2017) One-pot conversion of oil palm empty fruit bunch and mesocarp fiber biomass to levulinic acid and upgrading to ethyl levulinate via indium trichloride-ionic liquids. J Clean Prod 168:1251–1261
Abdul Khalil H, Siti Alwani M, Ridzuan R, Kamarudin H, Khairul A (2008) Chemical composition, morphological characteristics, and cell wall structure of Malaysian oil palm fibers. Polym Plast Technol Eng 47:273–280
Yang HP, Yan R, Chen HP, Lee DH, Liang DT, Zheng CG (2006) Pyrolysis of palm oil wastes for enhanced production of hydrogen rich gases. Fuel Process Technol 87:935–942
Yang HP, Yan R, Chin T, Liang DT, Chen HP, Zheng CG (2004) Thermogravimetric analysis−Fourier transform infrared analysis of palm oil waste pyrolysis. Energy Fuels 18:1814–1821
Adinata D, Wan Daud WMA, Aroua MK (2007) Production of carbon molecular sieves from palm shell based activated carbon by pore sizes modification with benzene for methane selective separation. Fuel Process Technol 88:599–605
Guo J, Xu WS, Chen YL, Lua AC (2005) Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4. J Colloid Interface Sci 281:285–290
Lim WC, Srinivasakannan C, Balasubramanian N (2010) Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J Anal Appl Pyrolysis 88:181–186
Wahi R, Ngaini Z, Usun Jok V (2009) Removal of mercury, lead and copper from aqueous solution by activated carbon of palm oil empty fruit bunch. World Appl Sci J 5:84–91
Daud WMAW, Ali WSW, Sulaiman MZ (2000) The effects of carbonization temperature on pore development in palm-shell-based activated carbon. Carbon 38:1925–1932
Yang KB, Peng JH, Srinivasakannan C, Zhang LB, Xia HY, Duan XH (2010) Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour Technol 101:6163–6169
Meng LY, Park SJ (2010) Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J Colloid Interface Sci 352:498–503
Choi MK, Ryoo R (2007) Mesoporous carbons with KOH activated framework and their hydrogen adsorption. J Mater Chem 17:4204–4209
Wang JC, Kaskel S (2012) KOH activation of carbon-based materials for energy storage. J Mater Chem 22:23710–23725
Arami-Niya A, Daud WMAW, Mjalli FS (2011) Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem Eng Res Des 89:657–664
Rodríguez-Reinoso F, Molina-Sabio M (1992) Activated carbons from lignocellulosic materials by chemical and/or physical activation: an overview. Carbon 30:1111–1118
Song M, Jin BS, Xiao R, Yang L, Wu YM, Zhong ZP, Huang YJ (2013) The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass Bioenergy 48:250–256
Budinova T, Ekinci E, Yardim F, Grimm A, Björnbom E, Minkova V, Goranova M (2006) Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process Technol 87:899–905
Herawan SG, Hadi MS, Ayob MR, Putra A (2013) Characterization of activated carbons from oil-palm shell by CO2 activation with no holding carbonization temperature. Sci World J 2013:1–6
Lee T, Zubir ZA, Jamil FM, Matsumoto A, Yeoh FY (2014) Combustion and pyrolysis of activated carbon fibre from oil palm empty fruit bunch fibre assisted through chemical activation with acid treatment. J Anal Appl Pyrolysis 110:408–418
Lua AC, Guo J (2001a) Microporous oil-palm-shell activated carbon prepared by physical activation for gas-phase adsorption. Langmuir 17:7112–7117
Molina-Sabio M, Gonzalez MT, Rodriguez-Reinoso F, Sepúlveda-Escribano A (1996) Effect of steam and carbon dioxide activation in the micropore size distribution of activated carbon. Carbon 34:505–509
Hidayu AR, Mohamad NF, Matali S, Sharifah ASAK (2013) Characterization of activated carbon prepared from oil palm empty fruit bunch using BET and FT-IR techniques. Procedia Eng 68:379–384
González JF, Román S, González-García CM, Nabais JMV, Ortiz AL (2009) Porosity development in activated carbons prepared from walnut shells by carbon dioxide or steam activation. Ind Eng Chem Res 48:7474–7481
Plaza MG, González AS, Pis JJ, Rubiera F, Pevida C (2014) Production of microporous biochars by single-step oxidation: effect of activation conditions on CO2 capture. Appl Energy 114:551–562
Román S, González JF, González-García CM, Zamora F (2008) Control of pore development during CO2 and steam activation of olive stones. Fuel Process Technol 89:715–720
Baby R, Saifullah B, Hussein MZ (2019) Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanosc Res Lett 14:341
Sevilla M, Maciá-Agulló JA, Fuertes AB (2011) Hydrothermal carbonization of biomass as a route for the sequestration of CO2: chemical and structural properties of the carbonized products. Biomass Bioenergy 35:3152–3159
Guo J, Lua AC (2001) Experimental and kinetic studies on pore development during CO2 activation of oil-palm-shell char. J Porous Mat 8:149–157
Ahmad MA, Daud WMAW, Aroua MK (2007) Synthesis of carbon molecular sieves from palm shell by carbon vapor deposition. J Porous Mat 14:393–399
Thostenson E, Chou T (1999) Microwave processing: fundamentals and applications. Compos Part A Appl S 30:1055–1071
Foo KY, Hameed BH (2011c) Preparation and characterization of activated carbon from pistachio nut shells via microwave-induced chemical activation. Biomass Bioenergy 35:3257–3261
Lin BJ, Chen WH (2015) Sugarcane bagasse pyrolysis in a carbon dioxide atmosphere with conventional and microwave-assisted heating. Front Energy Res 3:1–9
Menéndez J, Menéndez E, Garcia A, Parra J, Pis J (1999) Thermal treatment of active carbons: a comparison between microwave and electrical heating. J Microw Power Electromagn Energy 34:137–143
Kundu A, Gupta BS, Hashim M, Sahu J, Mujawar M, Redzwan G (2015) Optimisation of the process variables in production of activated carbon by microwave heating. RSC Adv 5:35899–35908
Guo J, Lua AC (2003a) Textural and chemical properties of adsorbent prepared from palm shell by phosphoric acid activation. Mater Chem Phys 80:114–119
Guo J, Luo Y, Lua AC, Chi RA, Chen YL, Bao XT, Xiang SX (2007) Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 45:330–336
Lua AC, Lau FY, Guo J (2006) Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J Anal Appl Pyrolysis 76:96–102
Guo J, Lua AC (2003b) Adsorption of sulphur dioxide onto activated carbon prepared from oil-palm shells with and without pre-impregnation. Sep Purif Technol 30:265–273
Lua AC, Guo J (2001b) Adsorption of sulfur dioxide on activated carbon from oil-palm waste. J Environ Eng 127:895–901
Guo J, Gui B, Xiang SX, Bao XT, Zhang HJ, Lua AC (2008) Preparation of activated carbons by utilizing solid wastes from palm oil processing mills. J Porous Mat 15:535–540
Guo J, Lua AC (2002a) Characterization of adsorbent prepared from oil-palm shell by CO2 activation for removal of gaseous pollutants. Mater Lett 55:334–339
Guo J, Lua AC (2002b) Microporous activated carbons prepared from palm shell by thermal activation and their application to sulfur dioxide adsorption. J Colloid Interface Sci 251:242–247
Sumathi S, Bhatia S, Lee KT, Mohamed AR (2009) Optimization of microporous palm shell activated carbon production for flue gas desulphurization: experimental and statistical studies. Bioresour Technol 100:1614–1621
Sivakumar VM, Kok KL, Mohamed A (2010) Synthesis of carbon molecular sieve from palm shell using deposition of polyfurfuryl alcohol. J Korean Chem Soc 54:323–328
Alam MZ, Ameem ES, Muyibi SA, Kabbashi NA (2009) The factors affecting the performance of activated carbon prepared from oil palm empty fruit bunches for adsorption of phenol. Chem Eng J 155:191–198
Jia QP, Lua AC (2008a) Concentration-dependent branched pore kinetic model for aqueous phase adsorption. Chem Eng J 136:227–235
Lua AC, Jia QP (2009) Adsorption of phenol by oil–palm-shell activated carbons in a fixed bed. Chem Eng J 150:455–461
Jia QP, Lua AC (2008b) Effects of pyrolysis conditions on the physical characteristics of oil-palm-shell activated carbons used in aqueous phase phenol adsorption. J Anal Appl Pyrolysis 83:175–179
Ahmad M (2009) Preparation of carbon molecular sieves from palm shell: effect of benzene deposition conditions. Adsorption 15:489–495
Klose W, Rincón S (2007) Adsorption and reaction of NO on activated carbon in the presence of oxygen and water vapour. Fuel 86:203–209
Daud WMAW, Ahmad MA, Aroua MK (2007) Carbon molecular sieves from palm shell: effect of the benzene deposition times on gas separation properties. Sep Purif Technol 57:289–293
Ahmad MA, Daud WMAW, Aroua MK (2008) Adsorption kinetics of various gases in carbon molecular sieves (CMS) produced from palm shell. Colloids Surf A 312:131–135
Katesa J, Junpiromand S, Tangsathitkulchai C (2013) Effect of carbonization temperature on properties of char and activated carbon from coconut shell. Suranaree J Sci Technol 20:269–278
Loredo-Cancino M, Soto-Regalado E, Cerino-Córdova FJ, García-Reyes RB, García-León AM, Garza-González MT (2013) Determining optimal conditions to produce activated carbon from barley husks using single or dual optimization. J Environ Manag 125:117–125
Sevilla M, Mokaya R (2014) Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ Sci 7:1250–1280
Tang SH, Zaini MAA (2015) Potassium hydroxide activation of activated carbon: a commentary. Carbon Lett 16:275–280
Sangchoom W, Mokaya R (2015) Valorization of lignin waste: carbons from hydrothermal carbonization of renewable lignin as superior sorbents for CO2 and hydrogen storage. ACS Sustain Chem Eng 3:1658–1667
Hussein MZB, Rahman MBBA, Yahaya AHJ, Taufiq-Yap YH, Ahmad N (2001) Oil palm trunk as a raw material for activated carbon production. J Porous Mat 8:327–334
Shaarani FW, Hameed BH (2010) Batch adsorption of 2,4-dichlorophenol onto activated carbon derived from agricultural waste. Desalination 255:159–164
Al Bahri M, Calvo L, Gilarranz MA, Rodriguez JJ (2012) Activated carbon from grape seeds upon chemical activation with phosphoric acid: application to the adsorption of diuron from water. Chem Eng J 203:348–356
Kilic M, Apaydin-Varol E, Pütün AE (2011) Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: equilibrium, kinetics and thermodynamics. J Hazard Mater 189:397–403
Ahmadpour A, Do DD (1996) The preparation of active carbons from coal by chemical and physical activation. Carbon 34:471–479
Nowicki P, Pietrzak R, Wachowska H (2008) Siberian anthracite as a precursor material for microporous activated carbons. Fuel 87:2037–2040
Vargas AMM, Cazetta AL, Garcia CA, Moraes JCG, Nogami EM, Lenzi E, Costa WF, Almeida VC (2011) Preparation and characterization of activated carbon from a new raw lignocellulosic material: flamboyant (Delonix regia) pods. J Environ Manag 92:178–184
Basta AH, Fierro V, El-Saied H, Celzard A (2009) 2-Steps KOH activation of rice straw: an efficient method for preparing high-performance activated carbons. Bioresour Technol 100:3941–3947
Cao Q, Xie KC, Lv YK, Bao WR (2006) Process effects on activated carbon with large specific surface area from corn cob. Bioresour Technol 97:110–115
Ahmed Hared I, Dirion J, Salvador S, Lacroix M, Rio S (2007) Pyrolysis of wood impregnated with phosphoric acid for the production of activated carbon: kinetics and porosity development studies. J Anal Appl Pyrolysis 79:101–105
Gokce Y, Aktas Z (2014) Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl Surf Sci 313:352–359
Jagtoyen M, Derbyshire F (1998) Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 36:1085–1097
Yagmur E, Ozmak M, Aktas Z (2008) A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel 87:3278–3285
Zuo SL, Yang JX, Liu JL, Cai X (2009) Significance of the carbonization of volatile pyrolytic products on the properties of activated carbons from phosphoric acid activation of lignocellulosic material. Fuel Process Technol 90:994–1001
Dobele G, Rossinskaja G, Telysheva G, Meier D, Faix O (1999) Cellulose dehydration and depolymerization reactions during pyrolysis in the presence of phosphoric acid. J Anal Appl Pyrolysis 49:307–317
Jagtoyen M, Derbyshire F (1993) Some considerations of the origins of porosity in carbons from chemically activated wood. Carbon 31:1185–1192
Solum MS, Pugmire RJ, Jagtoyen M, Derbyshire F (1995) Evolution of carbon structure in chemically activated wood. Carbon 33:1247–1254
Molina-Sabio M, RodRíguez-Reinoso F, Caturla F, Sellés MJ (1995) Porosity in granular carbons activated with phosphoric acid. Carbon 33:1105–1113
Zuo SL, Xiao ZL, Yang JX (2012) Evolution of gaseous products from biomass pyrolysis in the presence of phosphoric acid. J Anal Appl Pyrolysis 95:236–240
Jagtoyen M, Thwaites M, Stencel J, McEnaney B, Derbyshire F (1992) Adsorbent carbon synthesis from coals by phosphoric acid activation. Carbon 30:1089–1096
Giraldo L, Moreno-Piraján JC (2012) Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. E- J Chem 9:938–948
Guo J, Lua AC (2002c) Textural and chemical characterizations of adsorbent prepared from palm shell by potassium hydroxide impregnation at different stages. J Colloid Interface Sci 254:227–233
Alhamed YA, Rather SU, El-Shazly AH, Zaman SF, Daous MA, Al-Zahrani AA (2015) Preparation of activated carbon from fly ash and its application for CO2 capture. Korean J Chem Eng 32:723–730
Tan ZQ, Chen GX, Zhu YW (2015) Carbon-based supercapacitors produced by the activation of graphene. In: Feng XL (ed) Nanocarbons for advanced energy storage, 1st edn. Wiley, Weinheim
Azargohar R, Dalai AK (2008) Steam and KOH activation of biochar: experimental and modeling studies. Micropor Mesopor Mater 110:413–421
Madhu R, Veeramani V, Chen SM (2014) Heteroatom-enriched and renewable banana-stem-derived porous carbon for the electrochemical determination of nitrite in various water samples. Sci Rep 4:1–8
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2003) Understanding chemical reactions between carbons and NaOH and KOH: an insight into the chemical activation mechanism. Carbon 41:267–275
Unur E (2013) Functional nanoporous carbons from hydrothermally treated biomass for environmental purification. Micropor Mesopor Mater 168:92–101
Hayashi J, Horikawa T, Takeda I, Muroyama K, Ani FN (2002) Preparing activated carbon from various nutshells by chemical activation with K2CO3. Carbon 40:2381–2386
Khalil H, Jawaid M, Firoozian P, Rashid U, Islam A, Akil HM (2013) Activated carbon from various agricultural wastes by chemical activation with KOH: preparation and characterization. J Biobased Mater Bio 7:708–714
Abechi S, Gimba C, Uzairu A, Dallatu Y (2013) Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res J Chem Sci 3:54–61
Deng H, Zhang GL, Xu XL, Tao GH, Dai JL (2010) Optimization of preparation of activated carbon from cotton stalk by microwave assisted phosphoric acid-chemical activation. J Hazard Mater 182:217–224
Maldhure AV, Ekhe JD (2011) Preparation and characterizations of microwave assisted activated carbons from industrial waste lignin for Cu(II) sorption. Chem Eng J 168:1103–1111
Calvo EG, Menéndez JÁ, Arenillas A (2011) Designing nanostructured carbon xerogels. Nanomaterials. InTech, Rejika
Chen YD, Huang MJ, Chen WQ, Huang B (2012) Adsorption of Cu (II) from aqueous solution using activated carbon derived from mangosteen peel. BioResources 7:4965–4975
Lillo-Ródenas MA, Lozano-Castelló D, Cazorla-Amorós D, Linares-Solano A (2001) Preparation of activated carbons from Spanish anthracite: ii. activation by NaOH. Carbon 39:751–759
Pietrzak R, Nowicki P, Kaźmierczak J, Kuszyńska I, Goscianska J, Przepiórski J (2014) Comparison of the effects of different chemical activation methods on properties of carbonaceous adsorbents obtained from cherry stones. Chem Eng Res Des 92:1187–1191
Kan YJ, Yue QY, Gao BY, Li Q (2015) Comparative study of dry-mixing and wet-mixing activated carbons prepared from waste printed circuit boards by NaOH activation. RSC Adv 5:105943–105951
Lozano-Castelló D, Calo JM, Cazorla-Amorós D, Linares-Solano A (2007) Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon 45:2529–2536
Lim WC, Srinivasakannan C, Al Shoaibi A (2015) Cleaner production of porous carbon from palm shells through recovery and reuse of phosphoric acid. J Clean Prod 102:501–511
Zaini MAA, Tan WM, Kamaruddin MJ, Setapar SHM, Yunus MAC (2014) Microwave-induced zinc chloride activated palm kernel shell for dye removal. Sains Malays 43:1421–1428
Hamad BK (2015) Preparation and characterization of activated carbon from oil palm shell activated by KOH. ZANCO J Pure Appl Sci 27:27–38
Hamad BK, Noor AM, Afida AR, Mohd Asri MN (2010) High removal of 4-chloroguaiacol by high surface area of oil palm shell-activated carbon activated with NaOH from aqueous solution. Desalination 257:1–7
Guo J, Lua AC (1999) Textural and chemical characterisations of activated carbon prepared from oil-palm stone with H2SO4 and KOH impregnation. Micropor Mesopor Mater 32:111–117
Mohammadi M, Hassani AJ, Mohamed AR, Najafpour GD (2010) Removal of rhodamine B from aqueous solution using palm shell-based activated carbon: adsorption and kinetic studies. J Chem Eng Data 55:5777–5785
Hernández-Montoya V, García-Servin J, Bueno-López JI (2012) Thermal treatments and activation procedures used in the preparation of activated carbons. Lignocellulosic precursors used in the synthesis of activated carbon-characterization techniques and applications in the wastewater treatment. InTech, Rejika
Ooi CH, Ang CL, Yeoh FY (2013) The properties of activated carbon fiber derived from direct activation from oil palm empty fruit bunch fiber. Adv Mater Res 686:109–117
Salman JM (2014) Optimization of preparation conditions for activated carbon from palm oil fronds using response surface methodology on removal of pesticides from aqueous solution. Arab J Chem 7:101–108
Salman JM, Njoku VO, Hameed BH (2011) Batch and fixed-bed adsorption of 2,4-dichlorophenoxyacetic acid onto oil palm frond activated carbon. Chem Eng J 174:33–40
Arami-Niya A, Daud WMAW, Mjalli FS, Abnisa F, Shafeeyan MS (2012) Production of microporous palm shell based activated carbon for methane adsorption: modeling and optimization using response surface methodology. Chem Eng Res Des 90:776–784
El-Hendawy AA (2005) Surface and adsorptive properties of carbons prepared from biomass. Appl Surf Sci 252:287–295
Arami-Niya A, Daud WMAW, Mjalli FS (2010) Production of palm shell-based activated carbon with more homogeniouse pore size distribution. J Appl Sci 10:3361–3366
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye using activated carbon prepared from oil palm shell: batch and fixed bed studies. Desalination 225:13–28
Salman JM, Hameed BH (2010) Effect of preparation conditions of oil palm fronds activated carbon on adsorption of bentazon from aqueous solutions. J Hazard Mater 175:133–137
Tan IAW, Hameed BH, Ahmad AL (2007) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127:111–119
Hu ZH, Srinivasan MP (2001) Mesoporous high-surface-area activated carbon. Micropor Mesopor Mater 43:267–275
Guo J, Lua AC (2000) Effect of heating temperature on the properties of chars and activated carbons prepared from oil palm stones. J Therm Anal Calorim 60:417–425
Houshmand A, Daud WMAW, Shafeeyan MS (2011) Exploring potential methods for anchoring amine groups on the surface of activated carbon for CO2 adsorption. Sep Sci Technol 46:1098–1112
Shafeeyan MS, Daud WMAW, Houshmand A, Arami-Niya A (2012) The application of response surface methodology to optimize the amination of activated carbon for the preparation of carbon dioxide adsorbents. Fuel 94:465–472
Abdulrasheed AA, Jalil AA, Triwahyono S, Zaini MAA, Gambo Y, Ibrahim M (2018) Surface modification of activated carbon for adsorption of SO2 and NOX: a review of existing and emerging technologies. Renew Sust Energy Rev 94:1067–1085
Quang DV, Hatton TA, Abu-Zahra MRM (2016) Thermally stable amine-grafted adsorbent prepared by impregnating 3-aminopropyltriethoxysilane on mesoporous silica for CO2 capture. Ind Eng Chem Res 55:7842–7852
Builes S, Vega LF (2013) Effect of immobilized amines on the sorption properties of solid materials: impregnation versus grafting. Langmuir 29:199–206
Yin CY, Aroua MK, Daud WMAW (2007) Impregnation of palm shell activated carbon with polyethyleneimine and its effects on Cd2+ adsorption. Colloids Surf A 307:128–136
Owlad M, Aroua MK, Daud WMAW (2010) Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresour Technol 101:5098–5103
Yin CY, Aroua MK, Daud WMAW (2009) Fixed-bed adsorption of metal ions from aqueous solution on polyethyleneimine-impregnated palm shell activated carbon. Chem Eng J 148:8–14
Boehm HP, Mair G, Stoehr T, De Rincón AR, Tereczki B (1984) Carbon as a catalyst in oxidation reactions and hydrogen halide elimination reactions. Fuel 63:1061–1063
Bota KB, Abotsi GMK (1994) Ammonia: a reactive medium for catalysed coal gasification. Fuel 73:1354–1357
Stöhr B, Boehm HP, Schlögl R (1991) Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon 29:707–720
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143–151
Plaza MG, Pevida C, Arias B, Fermoso J, Casal MD, Martín CF, Rubiera F, Pis JJ (2009) Development of low-cost biomass-based adsorbents for postcombustion CO2 capture. Fuel 88:2442–2447
Plaza MG, Pevida C, Martín CF, Fermoso J, Pis JJ, Rubiera F (2010) Developing almond shell-derived activated carbons as CO2 adsorbents. Sep Purif Technol 71:102–106
Pereira MFR, Soares SF, Órfão JJM, Figueiredo JL (2003) Adsorption of dyes on activated carbons: influence of surface chemical groups. Carbon 41:811–821
Mangun CL, Benak KR, Economy J, Foster KL (2001) Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 39:1809–1820
Mangun CL, DeBarr JA, Economy J (2001) Adsorption of sulfur dioxide on ammonia-treated activated carbon fibers. Carbon 39:1689–1696
Bezerra DP, Oliveira RS, Vieira RS, Cavalcante CL, Azevedo DCS (2011) Adsorption of CO2 on nitrogen-enriched activated carbon and zeolite 13X. Adsorption 17:235–246
Plaza MG, Pevida C, Arenillas A, Rubiera F, Pis JJ (2007) CO2 capture by adsorption with nitrogen enriched carbons. Fuel 86:2204–2212
Veawab A, Tontiwachwuthikul P, Chakma A (1999) Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions. Ind Eng Chem Res 38:3917–3924
Guo B, Chang LP, Xie KC (2006a) Study of the behavior of adsorbing CS2 by activated carbon. Fuel Process Technol 87:873–881
Guo B, Chang LP, Xie KC (2006b) Adsorption of carbon dioxide on activated carbon. J Nat Gas Chem 15:223–229
Xu XC, Song CS, Andresen JM, Miller BG, Scaroni AW (2002) Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 16:1463–1469
Park HJ, Suh MP (2013) Enhanced isosteric heat, selectivity, and uptake capacity of CO2 adsorption in a metal-organic framework by impregnated metal ions. Chem Sci 4:685–690
Li FR, Yi HH, Tang XL, Ning P, Yu QF, Kang DJ (2010) Adsorption of carbon dioxide by coconut activated carbon modified with Cu/Ce. J Rare Earth 28:334–337
Somy A, Mehrnia MR, Amrei HD, Ghanizadeh A, Safari M (2009) Adsorption of carbon dioxide using impregnated activated carbon promoted by zinc. Int J Greenh Gas Con 3:249–254
Son SJ, Choi JS, Choo KY, Song SD, Vijayalakshmi S, Kim TH (2005) Development of carbon dioxide adsorbents using carbon materials prepared from coconut shell. Korean J Chem Eng 22:291–297
Zou Y, Mata VG, Rodrigues AE (2001) Adsorption of carbon dioxide on chemically modified high surface area carbon-based adsorbents at high temperature. Adsorption 7:41–50
Haro M, Ruiz B, Andrade M, Mestre AS, Parra JB, Carvalho AP, Ania CO (2012) Dual role of copper on the reactivity of activated carbons from coal and lignocellulosic precursors. Micropor Mesopor Mater 154:68–73
Hosseini S, Bayesti I, Marahel E, Babadi FE, Abdullah LC, Choong TSY (2015) Adsorption of carbon dioxide using activated carbon impregnated with Cu promoted by zinc. J Taiwan Inst Chem E 52:109–117
Liu YY, Wang ZYU, Zhou HC (2012) Recent advances in carbon dioxide capture with metal-organic frameworks. Greenh Gases Sci Technol 2:239–259
Sumathi S, Bhatia S, Lee KT, Mohamed AR (2010) Selection of best impregnated palm shell activated carbon (PSAC) for simultaneous removal of SO2 and NOx. J Hazard Mater 176:1093–1096
Ng C, Marshall WE, Rao RM, Bansode RR, Losso JN (2003) Activated carbon from pecan shell: process description and economic analysis. Ind Crop Prod 17:209–217
Fisher Scientific (2020) Carbon dioxide (99.80%, 48L)-06-802-417. https://www.fishersci.ca/shop/products/scotty-transportable-pure-gases-mixtures-48l-size-restek-31/06802417. Accessed 4 Mar 2020
Sigma-Aldrich (2020) Sodium hydroxide (reagent grade, ≥98%, pellets (anhydrous))-S5881; Poly(ethyleneimine) solution (analytical standard, 50 % (w/v) in H2O)-P3143; Phosphoric acid (ACS reagent, ≥85 wt. % in H2O)-695017; Potassium hydroxide (≥85% KOH basis, pellets, white)-P1767; Sulfuric acid (ACS reagent, 95.0–98.0%)-258105; Zinc chloride (reagent grade, ≥98%)-208086; Potassium carbonate (ACS reagent, ≥99.0%)-209619; 2-Amino-2-methyl-1-propanol (~5% Water, technical grade, 90%)-A65182; 2-Amino-2-methyl-1,3-propanediol (≥99%)-A9754; 2-(methylamino)ethanol (≥98%)-471445; Ethanolamine (ACS reagent, ≥99.0%)-398136; Diethanolamine (reagent grade, ≥98.0%)-D8885; Ethylenediamine (ReagentPlus®, ≥99%)-E26266; Diethylenetriamine (ReagentPlus®, 99%)-D93856; Nickel(II) nitrate hexahydrate (crystals or chunks)-244074; Ammonium metavanadate (99%)-205559; Iron(III) nitrate nonahydrate (ACS reagent, ≥98%)-216828; Cerium(III) nitrate hexahydrate (99% trace metals basis)-238538; Barium oxide (97%)-288497; Magnesium oxide (ACS reagent, 97%)-243388; Copper(II) oxide (ACS reagent, ≥99.0%)-241741; Titanium(IV) oxide (ReagentPlus®, ≥99%)-14021; Cerium(IV) oxide (≥99.0%)-22390. https://www.sigmaaldrich.com/united-states.html. Accessed 4 Mar 2020
Peters M, Timmerhaus K (1991) Plant design and economics for chemical engineers. McGraw-Hill, New York
Lai JY, Ngu LH (2020) The production cost analysis of oil palm waste activated carbon: a pilot-scale evaluation. Greenh Gases Sci Technol 10:999–1026
Przepiórski J, Czyżewski A, Pietrzak R, Toyoda M, Morawski AW (2013) Porous carbon material containing CaO for acidic gas capture: preparation and properties. J Hazard Mater 263:353–360
Przepiórski J, Czyżewski A, Pietrzak R, Morawski AW (2013) MgO/CaO-loaded activated carbon for carbon dioxide capture: practical aspects of use. Ind Eng Chem Res 52:6669–6677
Przepiórski J, Czyżewski A, Pietrzak R, Tryba B (2013) MgO/CaO-loaded porous carbons for carbon dioxide capture. J Therm Anal Calorim 111:357–364
Bougie F, Fan XF (2018) Microwave regeneration of monoethanolamine aqueous solutions used for CO2 capture. Int J Greenh Gas Con 79:165–172
Working Group III of the Intergovernmental Panel on Climate Change (IPCC) (2005) Capture of CO2. In: Metz B, Davidson O, Coninck Hd, Loos M, Meyer L (eds) IPCC, 2005: IPCC special report on carbon dioxide capture and storage. Cambridge University Press, Cambridge, New York, pp 105–178
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF (2005) Oxy-fuel combustion technology for coal-fired power generation. Prog Energy Combust Sci 31:283–307
Singh R, Ram Reddy MK, Wilson S, Joshi K, Diniz da Costa JC, Webley P (2009) High temperature materials for CO2 capture. Energy Procedia 1:623–630
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare that there are no known financial, personal, or other conflicts of interest associated with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lai, J.Y., Ngu, L.H., Hashim, S.S. et al. Review of oil palm-derived activated carbon for CO2 capture. Carbon Lett. 31, 201–252 (2021). https://doi.org/10.1007/s42823-020-00206-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-020-00206-1