Abstract
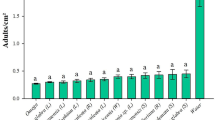
Parasitoids use herbivore-induced plant volatiles (HIPV´s) to locate their hosts. However, little is known about variations in HIPV´s production in genetically modified maize plants that are herbicide tolerant (singular event), insect resistant (Bt plants, singular event), or both herbicide tolerant and insect resistant, like staked events. We investigated the olfactory responses of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) to HIPV´s produced in maize (Zea mays) herbicide-tolerant and insect-resistant plants or their stacked events in response to damage caused by Spodoptera frugiperda during nighttime and daytime infestations. Real-time reverse-transcription PCR was used to assess whether the presence of one or more Bacillus thuringiensis (Bt) proteins and the time of induction of HIPV’s affected the expression of genes in plants under herbivore attack. The results showed that compounds were released during both nocturnal and diurnal infestations. However, some HIPV´s were released exclusively in infestations that started during the night by non-Bt plants and they were highly attractive to parasitoids. HIPV´s produced by non-Bt plants were more attractive to parasitoids than those released by Bt plants in infestations that started during the night. However, glyphosate-tolerant maize plants were more attractive to parasitoids than isogenic plants. The expression of the analyzed genes TPS10, TPS23, LOX10, and STC1 was higher in infestations that started during the night. In this study, we discuss the possible causes of the unresponsiveness of T. pretiosum females to HIPV´s produced by Bt maize.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Allmann S, Baldwin IT (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329:1075–1078
Anderson P, Alborn H (1999) Effects on oviposition behaviour and larval development of Spodoptera littoralis by herbivore-induced changes in cotton plants. Entomol Exp Appl 92:45–51
Arimura GI, Köpke S, Kunert M, Volpe V, David A, Brand P, Dabrowska P, Maffei ME, Boland W (2008) Effects of feeding Spodoptera littoralis on lima bean leaves: VI Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol 146:965–973
Batool A, Abdullah K, Mamoon-ur-Rashid M, Khattak MK, Abbas SS (2014) Effect of prey density on biology and functional response of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Pakistan J Zool 46:129–137
Christou P, Capell T, Kohli A, Gatehouse JA, Gatehouse AM (2006) Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci 11:302–308
Colazza S, Fucarino A, Peri E, Salerno G, Conti E et al (2004) Insect oviposition induces volatile emission in herbaceous plants that attracts the egg parasitoid Trissolcus basalis. J Exp Biol 207:47–53
Coll AMV, Jacobi VG, Fernandez PC, Luft AE, Virla EG, Hill JG, Catalán CAN (2019) Volatiles mediate host-selection in the corn hoppers Dalbulus maidis (Hemiptera: Cicadellidae) and Peregrinus maidis (Hemiptera: Delphacidae). Bull Entomol Res. 8:1–10
Crawley MJ (2013) The R Book, 2nd edn. JohnWiley & Sons, Chichester
D’auria JC, Pichersky E, Schaub A, Hansel A, Gershenzon J (2007) Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J 49:194–207
De Moraes CM, Mescher MC (2004) Biochemical crypsis in the avoidance of natural enemies by an insect herbivores. Proc Natl. Acad Sci USA 101:8993–8997
De Lange ES, Farnier K, Gaudillat B, Turlings TC (2016) Comparing the attraction of two parasitoids to herbivore-induced volatiles of maize and its wild ancestors, the teosintes. Chemoecology 26:33–44
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:29
Dean JM, De Moraes CM (2006) Effects of genetic modification on herbivore-induced volatiles from maize. J Chem Ecol 32:713–72
Dicke M (1999) Evolution of induced indirect defense of plants. In: Tollrian R, Harvell CD (eds) The Ecology and Evolution of Inducible Defenses. Princeton University Press, New Jersey, pp 62–88
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore induced plant volatiles: beyond the ‘cry for help.’ Trends Plant Sci 15:167–175
Dicke M, Sabelis MW, Takabayashi J, Bruni J, Posthumus MA (1990) Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol 16:3091–3118
Dicke M, Vanbeek TA, Posthumus MA, Bendom N, Vanbokhoven H, Degroot AE (1990) Isolation and identification of volatile kairomone that affects acarine predator–prey interactions—Involvement of host plant in its production. J Chem Ecol 16:381–396
Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902
Dudareva N, Negre F, Nagegowda D, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25:417–440
Fatouros NE, Bukovinszkine’Kiss G, Kalkers LA, Soler GR, Dicke M, et al (2005) Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entomol Exp Appl 115:207–215
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA et al (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7:e43607
Greenham K, McClung CR (2015) Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet 16:598–610
Head GP, Carroll MW, Evans SP, Rule DW, Willse AR, Clark TL et al (2017) Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: efficacy and resistance management. Pest Manag Sci 73:1883–1899
Hilker M, Meiners T (2006) Early herbivore alert: Insect eggs induce plant defense. J Chem Ecol 26:1379–1397
Hilker M, Meiners T (2010) How plants ‘“notice”’ attack by herbivores. Biol Ver 85:267–280
Himanen SJ, Nerg AM, Nissinen A, Pinto DM, Stewart CN Jr, Poppy GM, Holopainen JK (2009) Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytol 181:174–186
Hoballah ME, Turlings TCJ (2005) The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J Chem Ecol 31:2003–2018
Isaaa (2018) Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52. Ithaca, NY: ISAAA.
Jiao Y, Hu X, Peng Y, Wu K, Romeis J, Li Y (2018) Bt rice plants may protect neighboring non-Bt rice plants against the striped stem borer, Chilo suppressalis. Proc. R. Soc. B 285:20181283
Lawrence SD, Novak NG (2004) Maize genes induced by herbivory and volicitin. J Chem Ecol 30:2543–2557
Leppik E, Frérot B (2014) Maize field odorscape during the oviposition flight of the European corn borer. Chemoecology 24:221
Liu Q, Romeis J, Yu H et al (2015) Bt rice does not disrupt the host-searching behavior of the parasitoid Cotesia chilonis. Sci Rep 5:15295
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)). Method Methods 25:402–8
Mérey G, Veyrat N, Mahuku G, Valdez RL, Turlings TCJ, D’Alessandro M (2011) Dispensing synthetic green leaf volatiles in maize fields increases the release of sesquiterpenes by the plants, but has little effect on the attraction of pest and beneficial insects. Phytochem 72:1838
Montezano DG, Specht A, Sosa-Gómez DR, Roque-Specht VF, Sousa-Silva JC, Paula-Moraes SV, Peterson JA, Hunt TE (2018) Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. African Entomol 26:286–300
Moraes MC, Laumann RA, Aquino MF, Paula DP, Borges M (2011) Effect of Bt genetic engineering on indirect defense in cotton via a tritrophic interaction. Transgenic Res 20:99–107
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88:628–667
Mumm R, Tiemann T, Varama M, Hilker M (2005) Choosy egg parasitoids: Specificity of oviposition-induced pine volatiles exploited by an egg parasitoid of pine sawflies. Entomol Exp Appl 115:217–225
Naranjo-Guevara N, Peñaflor MFGV, Cabezas-Guerrero MF, Bento JMS (2017) Nocturnal herbivore-induced plant volatiles attract the generalist predatory earwig Doru luteipes. Scudder Sci Nat 104:77
Nascimento PT, Von Pinho RG, Fadini MAM, Souza CSF, Valicente FH (2020) Does singular and stacked corn affect choice behavior for oviposition and feed in Spodoptera frugiperda (Lepidoptera: Noctuidae)? Neotrop Entomol 49:302–310
Nemchenko A, Kunze S, Feussner I, Kolomiets M (2006) Duplicate maize 13- lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J Exp Botany 57:3767–3779
Paré PW, Tumlinson JH (1997) De Novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167
Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167
Peñaflor MFGV, Erb M, Miranda LA, Werneburg AG, Bento JMS (2011) Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol 37:1304–1313
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
R Core Team (2014) A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Schmelz EA, Alborn HT, Banchio E, Tumlinson JH (2003) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673
Schmelz EA, LeClere S, Carroll MJ, Alborn HT, Teal PE (2007) Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol 144:793–805
Schnee C, Köllner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-BFarnesene, (E)-Nerolidol, and (E, E)-Farnesol after herbivore damage. Plant Physiol 13:2049–2060
Schuler TH, Potting RPJ, Denholm I, Poppy GM (1999) Parasitoid behaviour and Bt plants. Nature 400:825–826
Shen B, Zheng Z, Dooner HK (2000) A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: characterization of wild-type and mutant alleles. Proc Natl Acad Sci U S A 97:14807–14812
Signoretti AGC, Penaflor MFGV, Moreira LSD, Noronha NC, Bento JMS (2012) Diurnal and nocturnal herbivore induction on maize elicit different innate response of the fall armyworm parasitoid, Campoletis flavicincta. J Pest Sci 85:101–107
Smith RM, Marshall JA, Davey MR, Lowe KC, Power JB (1996) Comparison of volatiles and waxes in leaves of genetically engineered tomatoes. Phytochemistry 43:753–758
Storer NP, Kubiszak ME, Ed King J, Thompson GD, Santos AC (2012) Status of resistance to Bt maize in Spodoptera frugiperda: lessons from Puerto Rico. J Invertebr Pathol 110: 294-300.
Tamiru A, Bruce TJA, Woodcock CM, Caulfield JC, Midega CAO, Ogol CKPO, Mayon P, Birkett MA, Pickett JA, Khan ZR (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol Lett 14:1075–1083
Tellez-Rodriguez P, Raymond B, Morán-Bertot I, Rodríguez-Cabrera L, Wright DJ, Borroto CG et al (2014) Strong oviposition preference for Bt over non-Bt maize in Spodoptera frugiperda and its implications for the evolution of resistance. BMC Biology 12:48
Ton J, D’Alessandro MD, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J Vancouver 49:16–26
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Ann Rev Entomol 63(1):433–452
Turlings TCJ, Tumlinson JH (1992) Systemic release of chemical signals by herbivore-injured corn. Proc Natl Acad Sci USA 89:8399–8402
Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207:146–152
Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH (2000) Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: isolation and bioactivity. J Chem Ecol 92:189–202
Turlings TC, Jeanbourquin PM, Held M, Degen T (2005) Evaluating the induced-odour emission of a Bt maize and its attractiveness to parasitic wasps. Transgenic Res 14:807–816
Valicente FH, Barreto MR (2003) Bacillus thuringiensis survey in Brazil: geographical distribution and insecticidal activity against Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop Entomol 32:639–644
Vandenborre G, Miersch O, Hause B, Smagghe G, Wasternack C, Van Damme EJM (2009) Spodoptera littoralis-induced lectin expression in tobacco. Plant Cell Physiol 50:1142–1155
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol 37:141–172
Vinson SB (1998) The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11:79–96
Wang X, Liu Q, Meissle M, Peng Y, Wu K, Romeis J, Li Y (2018) Bt rice could provide ecological resistance against nontarget planthoppers. Plant Biotechnol J 16(10):1748–1755. https://doi.org/10.1111/pbi.12911
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann Bot 111:1021–1058
Winkler K, Wäckers FL, Kaufman LV, Larraz V, van Lenteren JC (2009) Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol Control 50:299–306
Yan F, Bengtsson M, Anderson P, Ansebo L, Xu C, Witzgall P (2004) Antennal response of cotton bollworm (Helicoverpa armigera) to volatiles in transgenic Bt cotton. J Appl Entomol 128:354–357
Acknowledgements
The authors would like to thank the Postgraduate Program in Agronomy/Phytotechnics, for the scientific support to the student. Thanks to Embrapa Maize and Sorghum and Federal University of São João Del Rei, for the structure provided for the experiment. Thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for the financial support.
Funding
This work was supported by the National Council for Scientific and Technological Development. None of the funding bodies played any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
PTN planned, designed, and executed experimental work. MSR contributed with executed experimental. JOFM contributed with GC-MS analysis. Beatriz A. Barros contributed with RT-qPCR analysis. MAMF conducted data analyses. PTN wrote the manuscript. FHV, MAMF, CSFS, and RGVP reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Jarmo K. Holopainen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nascimento, P.T., Fadini, M.A.M., Rocha, M.S. et al. Response of Trichogramma pretiosum females (Hymenoptera: Trichogrammatidae) to herbivore-induced Bt maize volatiles. Arthropod-Plant Interactions 15, 107–125 (2021). https://doi.org/10.1007/s11829-020-09801-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-020-09801-5