Abstract
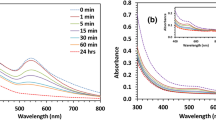
4-iodophenylboronic acid (IPBA) ligated luminescent gold cluster was synthesized by mixing an aqueous solution of IBPA and polyvinylpyrrolidone stabilized gold cluster (Au:PVP) in water at room temperature through chemisorption of iodine on gold nano surface. Transmission Electron microscopy (TEM) and matrix assisted laser desorption ionization (MALDI) analysis revealed that the size of these Au-clusters (1.4±0.2 nm) remain unchanged without any noticeable aggregation during synthesis. Owing to the formation of excimer between aryl moieties grafted over Au surface, the cluster exhibit strong emission peak at 335 nm. This luminescent gold cluster is used for sensing different saccharides in water at physiological pH through quenching of excimer emission peak. This strong excimer emission is significantly quenched in presence of saccharides through interaction with boronic acid moieties. The selectivity for different saccharides follows the order: fructose > galactose > maltose > glucose ~ ribose > sorbitol with hight affinity for fructose (KSV = 1.54 × 104 M−1) with Limit of Detection (LOD) of 100 μM.
Graphical Abstract








Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available in the article and in the supplementary materials.
References
Patel V (2019) Molecular nutrition: Carbohydrates. Academic Press, Cambridge
Popkin BM, Hawkes C (2016) Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol 4:174–186. https://doi.org/10.1016/S2213-8587(15)00419-2
Vashist SK (2012) Non-invasive glucose monitoring technology in diabetes management: a review. Anal Chim Acta 750:16–27. https://doi.org/10.1016/j.aca.2012.03.043
D’Auria S, Dicesare SN, Gryczynski Z, Gryczynski I, Rossi M, Lakowicz JR (2000) A thermophilic apoglucose dehydrogenase as nonconsuming glucose Sensor. Biochem Bioph Res Co 274:727–731. https://doi.org/10.1006/bbrc.2000.3172
Noiphung J, Songjaroen T, Dungchai W, Henry CS, Chailapakul O, Laiwattanapaisal W (2013) Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal Chim Acta 788:39–45. https://doi.org/10.1016/j.aca.2013.06.021
Rabinovitch B, March WF, Adams RL (1982) Noninvasive glucose monitoring of the aqueous humor of the eye: Part I. Measurement of very small optical rotations. Diabetes Care 5:254–258. https://doi.org/10.2337/diacare.5.3.254
Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, Robinson PL (1992) Noninvasive glucose monitoring in diabetic patients: a preliminary evaluation. Clin Chem 38:1618–1622
Palazzo G, Facchini L, Mallardi A (2012) Colorimetric detection of sugars based on gold nanoparticle formation. Sensor Actuat B-Chem 161:366–371. https://doi.org/10.1016/j.snb.2011.10.046
Caol H, Heagy MD (2004) Fluorescent chemosensors for carbohydrates: a decade’s worth of bright spies for saccharides in review. J Fluoresc 14:569–584. https://doi.org/10.1023/B:JOFL.0000039344.34642.4c
Zhang Y, He Z, Li G (2010) A novel fluorescent vesicular sensor for saccharides based on boronic acid–diol interaction. Talanta 81:591–596. https://doi.org/10.1016/j.talanta.2009.12.041
Hosseinzadeh R, Mohadjerani M, Pooryousef M, Eslami A, Emami S (2015) A new boronic acid fluorescent sensor based on fluorene for monosaccharides at physiological pH. Spectrochim Acta A 144:53–60. https://doi.org/10.1016/j.saa.2015.02.066
Ni N, Laughlin S, Wang Y, Feng Y, Zheng Y, Wang B (2012) Probing the general time scale question of boronic acid binding with sugars in aqueous solution at physiological pH. Bioorg Med Chem 20:2957–2961. https://doi.org/10.1016/j.bmc.2012.03.014
Jin S, Zhu C, Li M, Wang B (2009) Identification of the first fluorescent α-amidoboronic acids that change fluorescent properties upon sugar binding. Bioorg Med Chem Lett 19:1596–1599. https://doi.org/10.1016/j.bmcl.2009.02.011
Yoon J, Czarnik AW (1992) Fluorescent chemosensors of carbohydrates. A means of chemically communicating the binding of polyols in water based on chelation-enhanced quenching. J Am Chem Soc 114:5874–5875. https://doi.org/10.1021/ja00040a067
Sandanayake KRAS, Shinkai S (1994) Novel molecular sensors for saccharides based on the interaction of boronic acid and amines: saccharide sensing in neutral water. J Chem Soc Chem Commun:1083–1084. https://doi.org/10.1039/C39940001083
Shehab M, Ebrahim S, Soliman M Graphene quantum dots prepared from glucose as optical sensor for glucose. J Lumin 184:110–116. https://doi.org/10.1016/j.jlumin.2016.12.006
Wu W, Zhou T, Berliner A, Banerjee P, Zhou S (2010) Glucose-mediated assembly of phenylboronic acid modified CdTe/ZnTe/ZnS quantum dots for intracellular glucose probing. Angew Chem Int Ed 49:6554–6558. https://doi.org/10.1002/anie.201001508
Feng D-Q, Chen M, Liu G, Zhu W, Sun W, Zhu R, Wang W (2015) A novel resonance light scattering sensing for glucose based on the conversion of gold nanoclusters into gold nanoparticles. Sensor Actuat B-Chem 219:133–138. https://doi.org/10.1016/j.snb.2015.05.019
Cui M, Zhao Y, Song Q (2014) Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters. Trends Anal Chem 57:73–82. https://doi.org/10.1016/j.trac.2014.02.005
Chansuvarn W, Tuntulani T, Imyim A (2015) Colorimetric detection of mercury (II) based on gold nanoparticles, fluorescent gold nanoclusters and other gold-based nanomaterials. Trends Anal Chem 65:83–96. https://doi.org/10.1021/ac1021503
Huang Y, Fuksman L, Zhang J (2018) Luminescence mechanisms of ultrasmall gold nanoparticles. Dalton Trans 47:6267–6273. https://doi.org/10.1039/C8DT00420J
Yu Y, Luo Z, Chevrier DM, Leong DT, Zhang P, Jiang D, Xie J (2014) Identification of a highly luminescent Au22(SG)18 nanocluster. J Am Chem Soc 136:1246–1249. https://doi.org/10.1021/ja411643u
Pyo K, Thanthirige VD, Yoon SK, Ramakrishna G, Lee D (2016) Enhanced luminescence of Au22(SG)18 nanoclusters via rational surface engineering. Nanoscale 8:20008–20016. https://doi.org/10.1039/C6NR07660B
Maity P, Takano S, Yamazoe S, Wakabayashi T, Tsukuda T (2013) Binding motif of terminal alkynes on gold clusters. J Am Chem Soc 135:9450–9457. https://doi.org/10.1021/ja401798z
Ito S, Takano S, Tsukuda T (2019) Alkynyl-protected Au22(C≡CR)18 clusters featuring new interfacial motifs and R-dependent photoluminescence. J Phys Chem Lett 10:6892–6896. https://doi.org/10.1021/acs.jpclett.9b02920
Narouz MR, Takano S, Lummis PA, Levchenko TI, Nazemi A, Kaappa S, Malola S, Yousefalizadeh G, Calhoun LA, Stamplecoskie KG, Häkkinen H, Tsukuda T, Crudden CM (2019) Robust, Highly Luminescent Au13 superatoms protected by N-heterocyclic carbenes. J Am Chem Soc 141:14997–15002. https://doi.org/10.1021/jacs.9b07854
Rajamanikandan R, Ilanchelian M (2018) Protein-localized bright-red fluorescent gold nanoclusters as cyanide-selective colorimetric and fluorometric nanoprobes. ACS Omega 3:14111–14118. https://doi.org/10.1021/acsomega.8b02044
Zheng J, Petty JT, Dickson RM (2003) High quantum yield blue emission from water-soluble Au8 nanodots. J Am Chem Soc 125:7780–7781. https://doi.org/10.1021/ja035473v
Mathew A, Sajanlal PR, Pradeep T (2012) Selective visual detection of TNT at the sub-zeptomole level. Angew Chem Int Ed 51:9596–9600. https://doi.org/10.1002/anie.201203810
Kumar PP, Kaur N, Shanavas A, Neelakandan PP (2020) Nanomolar detection of biothiols via turn-ON fluorescent indicator displacement. Analyst 145:851–857. https://doi.org/10.1039/C9AN02222H
Yen Y-T, Chen T-Y, Chen C-Y, Chang C-L, Chyueh S-C, Chang H-T (2019) A photoluminescent colorimetric probe of bovine serum albumin-stabilized gold nanoclusters for new psychoactive substances: cathinone drugs in seized street samples. Sensors 19:3554–3564. https://doi.org/10.3390/s19163554
Luo QJ, Li ZG, Lai JH, Li FQ, Qiu P, Wang XL (2017) An on–off–on gold nanocluster-based fluorescent probe for sensitive detection of organophosphorus pesticides. RSC Adv 7:55199–55205. https://doi.org/10.1039/C7RA11835J
Goswami U, Sahoo AK, Chattopadhyay A, Ghosh SS (2018) In Situ synthesis of luminescent Au nanoclusters on a bacterial template for rapid detection, quantification, and distinction of kanamycin-resistant bacteria. ACS Omega 3:6113–6119. https://doi.org/10.1021/acsomega.8b00504
Maity P, Sasai K, Dhital RN, Sakai H, Hasobe T, Sakurai H (2020) Excimer formation of aryl iodides chemisorbed on gold nanoparticles for the significant enhancement of photoluminescence. J Phys Chem Lett 11:1199–1203. https://doi.org/10.1021/acs.jpclett.9b03557
Chavda N, Trivedi A, Thakarda J, Agrawal YK, Maity P (2016) Size specific activity of polymer stabilized gold nanoparticles for transfer hydrogenation catalysis. Catal Lett 146:1331–1339. https://doi.org/10.1007/s10562-016-1760-3
Tsunoyama H, Tsukuda T (2009) Magic numbers of gold clusters stabilized by PVP. J Am Chem Soc 131:18216–18217. https://doi.org/10.1021/ja908188f
Sarina S, Waclawik ER, Zhu H (2013) Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem 15:1814–1833. https://doi.org/10.1039/C3GC40450A
Thomas SW, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386. https://doi.org/10.1021/cr0501339
Funding
This work was supported by Council of Scientific & Industrial Research (CSIR, New Delhi) (Project no. 01(2873)/17/EMR-II).
Author information
Authors and Affiliations
Contributions
JT, PD, SB synthesized and characterized the nanocomposite and did all the sensing studies using spectrofluoremetry, SB performed MALDI mass measurements, JK and PM conceptualized the work and wrote the paper.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2794 kb)
Rights and permissions
About this article
Cite this article
Thakarda, J., Dave, P., Bhowmik, S. et al. 4-Iodophenylboronic Acid Stabilized Gold Cluster as a New Fluorescent Chemosensor for Saccharides Based on Excimer Emission Quenching. J Fluoresc 31, 447–454 (2021). https://doi.org/10.1007/s10895-020-02672-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02672-2