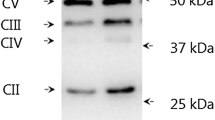
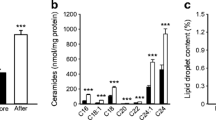
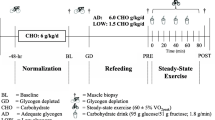
Abstract
Fasting rapidly (≤ 6 h) activates mitochondrial biogenic pathways in rodent muscle, an effect that is absent in human muscle following prolonged (10–72 h) fasting. We tested the hypotheses that fasting-induced changes in human muscle occur shortly after food withdrawal and are modulated by whole-body energetic stress. Vastus lateralis biopsies were obtained from ten healthy males before, during (4 h), and after (8 h) two supervised fasts performed with (FAST+EX) or without (FAST) 2 h of arm ergometer exercise (~ 400 kcal of added energy expenditure). PGC-1α mRNA (primary outcome measure) was non-significantly reduced (p = 0.065 [ηp2 = 0.14]) whereas PGC-1α protein decreased (main effect of time: p < 0.01) during both FAST and FAST+EX. P53 acetylation increased in both conditions (main effect of time: p < 0.01) whereas ACC and SIRT1 phosphorylation were non-significantly decreased (both p < 0.06 [ηp2 = 0.15]). Fasting-induced increases in NFE2L2 and NRF1 protein were observed (main effects of time: p < 0.03), though TFAM and COXIV protein remained unchanged (p > 0.05). Elevating whole-body energetic stress blunted the increase in p53 mRNA, which was apparent during FAST only (condition × time interaction: p = 0.04). Select autophagy/mitophagy regulators (LC3BI, LC3BII, BNIP3) were non-significantly reduced at the protein level (p ≤ 0.09 [ηp2 > 0.13]) but the LC3II:I ratio was unchanged (p > 0.05). PDK4 mRNA (p < 0.01) and intramuscular triglyceride content in type IIA fibers (p = 0.04) increased similarly during both conditions. Taken together, human skeletal muscle signaling, mRNA/protein expression, and substrate storage appear to be unaffected by whole-body energetic stress during the initial hours of fasting.





Similar content being viewed by others
References
Aquilano K, Baldelli S, Pagliei B, Cannata SM, Rotilio G, Ciriolo MR (2013) p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid Redox Signal 18:386–399. https://doi.org/10.1089/ars.2012.4615
Bak AM, Møller AB, Vendelbo MH, Nielsen TS, Viggers R, Rungby J, Pedersen SB, Jørgensen JOL, Jessen N, Møller N (2016) Differential regulation of lipid and protein metabolism in obese vs. lean subjects before and after a 72-h fast. Am J Physiol-Endocrinol Metab 311:E224–E235. https://doi.org/10.1152/ajpendo.00464.2015
Bak AM, Vendelbo MH, Christensen B, Viggers R, Bibby BM, Rungby J, Jørgensen JOL, Møller N, Jessen N (2018) Prolonged fasting-induced metabolic signatures in human skeletal muscle of lean and obese men. PLoS One 13:e0200817. https://doi.org/10.1371/journal.pone.0200817
Bergstrom J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616
Birk JB, Wojtaszewski JFP (2006) Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle: differential AMPK trimer activation in muscle. J Physiol 577:1021–1032. https://doi.org/10.1113/jphysiol.2006.120972
Bloemberg D, Quadrilatero J (2012) Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7:e35273. https://doi.org/10.1371/journal.pone.0035273
Bloemberg D, Quadrilatero J (2019) Autophagy, apoptosis, and mitochondria: molecular integration and physiological relevance in skeletal muscle. Am J Phys Cell Phys 317:C111–C130. https://doi.org/10.1152/ajpcell.00261.2018
Bujak AL, Crane JD, Lally JS, Ford RJ, Kang SJ, Rebalka IA, Green AE, Kemp BE, Hawke TJ, Schertzer JD, Steinberg GR (2015) AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metab 21:883–890. https://doi.org/10.1016/j.cmet.2015.05.016
Cantó C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20:98–105. https://doi.org/10.1097/MOL.0b013e328328d0a4
Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. https://doi.org/10.1038/nature07813
Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J (2010) Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11:213–219. https://doi.org/10.1016/j.cmet.2010.02.006
Caton PW, Holness MJ, Bishop-Bailey D, Sugden MC (2011) PPARα–LXR as a novel metabolostatic signalling axis in skeletal muscle that acts to optimize substrate selection in response to nutrient status. Biochem J 437:521–530. https://doi.org/10.1042/BJ20110702
Cerda-Kohler H, Henríquez-Olguín C, Casas M, Jensen TE, Llanos P, Jaimovich E (2018) Lactate administration activates the ERK1/2, mTORC1, and AMPK pathways differentially according to skeletal muscle type in mouse. Phys Rep 6:e13800. https://doi.org/10.14814/phy2.13800
Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE (2000) AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab 279:E1202–E1206. https://doi.org/10.1152/ajpendo.2000.279.5.E1202
Chen Z-P, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK (2003) Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52:2205–2212. https://doi.org/10.2337/diabetes.52.9.2205
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Crilly MJ, Tryon LD, Erlich AT, Hood DA (2016) The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol 121:730–740. https://doi.org/10.1152/japplphysiol.00042.2016
de Lange P, Farina P, Moreno M, Ragni M, Lombardi A, Silvestri E, Burrone L, Lanni A, Goglia F (2006) Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. FASEB J 20:2579–2581. https://doi.org/10.1096/fj.06-6025fje
Dethlefsen MM, Bertholdt L, Gudiksen A, Stankiewicz T, Bangsbo J, van Hall G, Plomgaard P, Pilegaard H (2018) Training state and skeletal muscle autophagy in response to 36 h of fasting. J Appl Physiol 125:1609–1619. https://doi.org/10.1152/japplphysiol.01146.2017
Edgett BA, Bonafiglia JT, Baechler BL, Quadrilatero J, Gurd BJ (2016) The effect of acute and chronic sprint-interval training on LRP130, SIRT3, and PGC-1 α expression in human skeletal muscle. Phys Rep 4:e12879. https://doi.org/10.14814/phy2.12879
Edgett BA, Scribbans TD, Raleigh JP, Matusiak JBL, Boonstra K, Simpson CA, Perry CGR, Quadrilatero J, Gurd BJ (2016) The impact of a 48-h fast on SIRT1 and GCN5 in human skeletal muscle. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 41:953–962. https://doi.org/10.1139/apnm-2016-0130
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461. https://doi.org/10.1126/science.1196371
Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M (2009) Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol 106:929–934. https://doi.org/10.1152/japplphysiol.90880.2008
Gordon JW, Rungi AA, Inagaki H, Hood DA (2001) Selected contribution: effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 90:389–396. https://doi.org/10.1152/jappl.2001.90.1.389
Gurd BJ (2011) Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 36:589–597. https://doi.org/10.1139/h11-070
Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor coactivator 1 expression in muscle. Proc Natl Acad Sci 100:7111–7116. https://doi.org/10.1073/pnas.1232352100
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. https://doi.org/10.1146/annurev-genet-102808-114910
Hoeks J, van Herpen NA, Mensink M, Moonen-Kornips E, van Beurden D, Hesselink MKC, Schrauwen P (2010) Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes 59:2117–2125. https://doi.org/10.2337/db10-0519
Høgild ML, Gudiksen A, Pilegaard H, Stødkilde-Jørgensen H, Pedersen SB, Møller N, Jørgensen JOL, Jessen N (2019) Redundancy in regulation of lipid accumulation in skeletal muscle during prolonged fasting in obese men. Phys Rep 7:e14285. https://doi.org/10.14814/phy2.14285
Islam H, Bonafiglia JT, Turnbull PC, Simpson CA, Perry CGR, Gurd BJ (2019) The impact of acute and chronic exercise on Nrf2 expression in relation to markers of mitochondrial biogenesis in human skeletal muscle. Eur J Appl Physiol 120:149–160. https://doi.org/10.1007/s00421-019-04259-7
Islam H, Edgett BA, Bonafiglia JT, Shulman T, Ma A, Quadrilatero J, Simpson CA, Gurd BJ (2019) Repeatability of exercise-induced changes in mRNA expression and technical considerations for qPCR analysis in human skeletal muscle. Exp Physiol 104:407–420. https://doi.org/10.1113/EP087401
Jäger S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104:12017–12022. https://doi.org/10.1073/pnas.0705070104
Jamart C, Naslain D, Gilson H, Francaux M (2013) Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am J Physiol-Endocrinol Metab 305:E964–E974. https://doi.org/10.1152/ajpendo.00270.2013
Kulkarni SR, Donepudi AC, Xu J, Wei W, Cheng QC, Driscoll MV, Johnson DA, Johnson JA, Li X, Slitt AL (2014) Fasting induces nuclear factor E2-related factor 2 and ATP-binding cassette transporters via protein kinase A and sirtuin-1 in mouse and human. Antioxid Redox Signal 20:15–30. https://doi.org/10.1089/ars.2012.5082
Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z (2017) Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8:548. https://doi.org/10.1038/s41467-017-00520-9
Lange P, Moreno M, Silvestri E, Lombardi A, Goglia F, Lanni A (2007) Fuel economy in food-deprived skeletal muscle: signaling pathways and regulatory mechanisms. FASEB J 21:3431–3441. https://doi.org/10.1096/fj.07-8527rev
Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ (2010) Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol-Regul Integr Comp Physiol 298:R912–R917. https://doi.org/10.1152/ajpregu.00409.2009
Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, Suzuki CK (2013) Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol Cell 49:121–132. https://doi.org/10.1016/j.molcel.2012.10.023
Merry TL, Ristow M (2016) Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice: NFE2L2 and mitochondrial biogenesis. J Physiol 594:5195–5207. https://doi.org/10.1113/JP271957
Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O (2007) An increase in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in response to exercise is mediated by β-adrenergic receptor activation. Endocrinology 148:3441–3448. https://doi.org/10.1210/en.2006-1646
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111. https://doi.org/10.1091/mbc.e03-09-0704
Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW (2002) Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol 92:2475–2482. https://doi.org/10.1152/japplphysiol.00071.2002
Piantadosi CA, Carraway MS, Babiker A, Suliman HB (2008) Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory Factor-1. Circ Res 103:1232–1240. https://doi.org/10.1161/01.RES.0000338597.71702.ad
Pilegaard H, Saltin B, Neufer PD (2003) Effect of short-term fasting and refeeding on transcriptional regulation of metabolic genes in human skeletal muscle. Diabetes 52:657–662. https://doi.org/10.2337/diabetes.52.3.657
Preobrazenski N, Islam H, Drouin PJ, Bonafiglia JT, Tschakovsky ME, Gurd BJ (2019) A novel gravity-induced blood flow restriction model augments ACC phosphorylation and PGC-1α mRNA in human skeletal muscle following aerobic exercise: a randomized crossover study. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 45:641–649. https://doi.org/10.1139/apnm-2019-0641
Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta BBA - Mol Cell Res 1813:1269–1278. https://doi.org/10.1016/j.bbamcr.2010.09.019
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Schwalm C, Jamart C, Benoit N, Naslain D, Prémont C, Prévet J, Van Thienen R, Deldicque L, Francaux M (2015) Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J 29:3515–3526. https://doi.org/10.1096/fj.14-267187
Scribbans TD, Edgett BA, Vorobej K, Mitchell AS, Joanisse SD, Matusiak JBL, Parise G, Quadrilatero J, Gurd BJ (2014) Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLoS One 9:e98119. https://doi.org/10.1371/journal.pone.0098119
Stannard SR, Thompson MW, Fairbairn K, Huard B, Sachinwalla T, Thompson CH (2002) Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men. Am J Physiol-Endocrinol Metab 283:E1185–E1191. https://doi.org/10.1152/ajpendo.00108.2002
Stephens TJ, Chen Z-P, Canny BJ, Michell BJ, Kemp BE, McConell GK (2002) Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Am J Physiol-Endocrinol Metab 282:E688–E694. https://doi.org/10.1152/ajpendo.00101.2001
Tsintzas K, Jewell K, Kamran M, Laithwaite D, Boonsong T, Littlewood J, Macdonald I, Bennett A (2006) Differential regulation of metabolic genes in skeletal muscle during starvation and refeeding in humans: metabolic genes and starvation in humans. J Physiol 575:291–303. https://doi.org/10.1113/jphysiol.2006.109892
Tunstall RJ, Mehan KA, Hargreaves M, Spriet LL, Cameron-Smith D (2002) Fasting activates the gene expression of UCP3 independent of genes necessary for lipid transport and oxidation in skeletal muscleq. Biochem Biophys Res Commun 294(2):301–8. https://doi.org/10.1016/S0006-291X(02)00473-4
Vaziri H, Dessain SK, Eaton EN, Imai S-I, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107:149–159. https://doi.org/10.1016/S0092-8674(01)00527-X
Vendelbo MH, Clasen BFF, Treebak JT, Møller L, Krusenstjerna-Hafstrøm T, Madsen M, Nielsen TS, Stødkilde-Jørgensen H, Pedersen SB, Jørgensen JOL, Goodyear LJ, Wojtaszewski JFP, Møller N, Jessen N (2012) Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle. Am J Physiol-Endocrinol Metab 302:E190–E200. https://doi.org/10.1152/ajpendo.00207.2011
Wijngaarden MA, van der Zon GC, van Dijk KW, Pijl H, Guigas B (2013) Effects of prolonged fasting on AMPK signaling, gene expression, and mitochondrial respiratory chain content in skeletal muscle from lean and obese individuals. Am J Physiol-Endocrinol Metab 304:E1012–E1021. https://doi.org/10.1152/ajpendo.00008.2013
Wijngaarden MA, Bakker LEH, van der Zon GC, ’t Hoen PAC, van Dijk KW, Jazet IM, Pijl H, Guigas B (2014) Regulation of skeletal muscle energy/nutrient-sensing pathways during metabolic adaptation to fasting in healthy humans. Am J Physiol-Endocrinol Metab 307:E885–E895. https://doi.org/10.1152/ajpendo.00215.2014
Acknowledgments
We would like to thank all participants for their time and effort.
Funding
This work was supported by funding provided to B.J.G. from the Natural Sciences and Engineering Research Council of Canada (NSERC; grant no. 402635). H.I. was supported by a post-graduate scholarship (Doctoral) from NSERC.
Author information
Authors and Affiliations
Contributions
H.I., A.A., and B.J.G conceptualized and designed the study. H.I., A.A., C.A.S., and B.J.G. collected the data. H.I., A.A., J.T.B., F.A.R., N.P., A.M., J.Q., and B.J.G. contributed to data analysis and interpretation. H.I. drafted the first version of the manuscript. All authors contributed to manuscript revisions and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 15.9 kb)
Fig S1
Representative images for GAPDH and amido black staining for total protein. (PNG 78 kb)
Fig S2
NFE2L2 (A), LRP130 (B) and SIRT1 (C) mRNA expression in the vastus lateralis before (PRE), during (MID; 4 hours) and after (POST; 8 hours) an acute fast performed with (FAST+EX; grey boxes) or without (FAST; white boxes) 2-hours of low-intensity arm ergometer exercise (n=10). Note: Boxes, whiskers, horizontal lines, and crosses indicate interquartile ranges, min/max values, medians, and means, respectively (PNG 127 kb)
Fig S3
Expression of protein involved in intracellular signaling (A), mitochondrial biogenesis (B- D), and autophagy/mitophagy (E-I) in the vastus lateralis before (PRE), during (MID; 4 hours) and after (POST; 8 hours) an acute fast performed with (FAST+EX; grey boxes) or without (FAST; white boxes) 2-hours of low-intensity arm ergometer exercise (n = 10). Note: Boxes, whiskers, horizontal lines, and crosses indicate interquartile ranges, min/max values, medians, and means, respectively. Significant/near- significant main effects and interactions (two-way RM- ANOVA) are reported below graphs where appropriate. Symbols denote significant (* p < 0.05) or near- significant (# p < 0.10) difference versus PRE (Bonferroni post-hoc). (PNG 559 kb)
Rights and permissions
About this article
Cite this article
Islam, H., Amato, A., Bonafiglia, J.T. et al. Increasing whole-body energetic stress does not augment fasting-induced changes in human skeletal muscle. Pflugers Arch - Eur J Physiol 473, 241–252 (2021). https://doi.org/10.1007/s00424-020-02499-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02499-7