Abstract
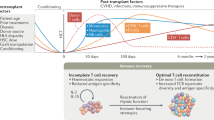
As the thymus represents the primary site of T-cell development, optimal thymic function is of paramount importance for the successful reconstitution of the adaptive immunity after allogeneic hematopoietic stem cell transplantation. Thymus involutes as part of the aging process and several factors, including previous chemotherapy treatments, conditioning regimen used in preparation to the allograft, occurrence of graft-versus-host disease, and steroid therapy that impair the integrity of the thymus, thus affecting its role in supporting T-cell neogenesis. Although the pathways governing its regeneration are still poorly understood, the thymus has a remarkable capacity to recover its function after damage. Measurement of both recent thymic emigrants and T-cell receptor excision circles is valuable tools to assess thymic output and gain insights on its function. In this review, we will extensively discuss available data on factors regulating thymic function after allogeneic hematopoietic stem cell transplantation, as well as the strategies and therapeutic approaches under investigation to promote thymic reconstitution and accelerate immune recovery in transplanted patients, including the use of cytokines, sex-steroid ablation, precursor T-cells, and thymus bioengineering. Although none of them is routinely used in the clinic, these approaches have the potential to enhance thymic function and immune recovery, not only in patients given an allograft but also in other conditions characterized by immune deficiencies related to a defective function of the thymus.



Similar content being viewed by others
References
Chabannon C, Kuball J, Bondanza A, Dazzi F, Pedrazzoli P, Toubert A, Ruggeri A, Fleischhauer K, Bonini C (2018) Hematopoietic stem cell transplantation in its 60s: a platform for cellular therapies. Sci Transl Med 10:eaap9630
Rocha V, Locatelli F (2008) Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplant 41:207–214
Locatelli F, Zecca M, Messina C, Rondelli R, Lanino E, Sacchi N, Uderzo C, Fagioli F, Conter V, Bonetti F, Favre C, Porta F, Giorgiani G, Pession A (2002) Improvement over time in outcome for children with acute lymphoblastic leukemia in second remission given hematopoietic stem cell transplantation from unrelated donors. Leukemia 16:2228–2237
Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W, Lang P, Sykora KW, Schrum J, Kremens B, Ehlert K, Albert MH, Meisel R, Matthes-Martin S, Gungor T, Holter W, Strahm B, Gruhn B, Schulz A, Woessmann W, Poetschger U, Zimmermann M, Klingebiel T (2015) Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol 33:1265–1274
Ballen KK, Gluckman E, Broxmeyer HE (2013) Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122:491–498
Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, Tiedemann K, Busca A, Chan KW, Kato S, Ortega J, Vowels M, Zander A, Souillet G, Oakill A, Woolfrey A, Pay AL, Green A, Garnier F, Ionescu I, Wernet P, Sirchia G, Rubinstein P, Chevret S, Gluckman E (2001) Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 97:2962–2971
Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D, Maiers M (2014) HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 371:339–348
Mancusi A, Ruggeri L, Velardi A (2016) Haploidentical hematopoietic transplantation for the cure of leukemia: from its biology to clinical translation. Blood 128:2616–2623
Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, Rondelli R, Lucarelli B, Brescia LP, Masetti R, Milano GM, Bertaina V, Algeri M, Pinto RM, Strocchio L, Meazza R, Grapulin L, Handgretinger R, Moretta A, Bertaina A, Moretta L (2017) Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood 130:677–685
Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L (2018) NK cells mediate a crucial graft-versus-leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol 39:577–590
McCurdy SR, Luznik L (2019) How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Blood 134:1802–1810
Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, Passweg J, Roosnek E (2008) Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol 30:425–437
Waller EK, Logan BR, Fei M, Lee SJ, Confer D, Howard A, Chandrakasan S, Anasetti C, Fernando SM, Giver CR (2019) Kinetics of immune cell reconstitution predict survival in allogeneic bone marrow and G-CSF-mobilized stem cell transplantation. Blood Adv 3:2250–2263
Le Blanc K, Barrett AJ, Schaffer M, Hagglund H, Ljungman P, Ringden O, Remberger M (2009) Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant 15:1108–1115
Wils EJ, van der Holt B, Broers AE, Posthumus-van Sluijs SJ, Gratama JW, Braakman E, Cornelissen JJ (2011) Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica 96:1846–1854
Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, Garnier F, Douek D, Gluckman E, Charron D, Toubert A (2002) A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood 99:1458–1464
Clave E, Rocha V, Talvensaari K, Busson M, Douay C, Appert ML, Rabian C, Carmagnat M, Garnier F, Filion A, Socie G, Gluckman E, Charron D, Toubert A (2005) Prognostic value of pretransplantation host thymic function in HLA-identical sibling hematopoietic stem cell transplantation. Blood 105:2608–2613
Clave E, Lisini D, Douay C, Giorgiani G, Busson M, Zecca M, Moretta F, Acquafredda G, Brescia LP, Locatelli F, Toubert A (2013) Thymic function recovery after unrelated donor cord blood or T-cell depleted HLA-haploidentical stem cell transplantation correlates with leukemia relapse. Front Immunol 4:54
Roux E, Dumont-Girard F, Starobinski M, Siegrist CA, Helg C, Chapuis B, Roosnek E (2000) Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood 96:2299–2303
Krenger W, Blazar BR, Hollander GA (2011) Thymic T-cell development in allogeneic stem cell transplantation. Blood 117:6768–6776
Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, Gress RE (2005) Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 115:930–939
Gonzalez-Vicent M, Molina B, Deltoro N, Sevilla J, Vicario JL, Castillo A, Ramirez M, Diaz MA (2017) Donor age matters in T-cell depleted haploidentical hematopoietic stem cell transplantation in pediatric patients: faster immune reconstitution using younger donors. Leuk Res 57:60–64
Baron F, Storer B, Maris MB, Storek J, Piette F, Metcalf M, White K, Sandmaier BM, Maloney DG, Storb R, Boeckh M (2006) Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant 12:1176–1187
Dalle JH, Balduzzi A, Bader P, Lankester A, Yaniv I, Wachowiak J, Pieczonka A, Bierings M, Yesilipek A, Sedlacek P, Ifversen M, Sufliarska S, Toporski J, Glogova E, Poetschger U, Peters C (2018) Allogeneic stem cell transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 BFM and 2007 International BFM Studies: impact of disease risk on outcomes. Biol Blood Marrow Transplant 24:1848–1855
Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, Weissinger E (2016) Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol 7:507
Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C, Carmagnat M, Esperou H, Ribaud P, Devergie A, Guardiola P, Vexiau P, Charron D, Gluckman E, Socie G (2001) Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol 115:630–641
Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Luo X, Curtsinger J, Mehta RS, Warlick E, Cooley SA, Blazar BR, Miller JS, Weisdorf D, Wagner JE, Verneris MR (2018) Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv 2:909–922
Castillo N, Garcia-Cadenas I, Diaz-Heredia C, Martino R, Barba P, Ferra C, Canals C, Elorza I, Olive T, Badell I, Sierra J, Valcarcel D, Querol S (2016) Cord blood units with high CD3(+) cell counts predict early lymphocyte recovery after in vivo T cell-depleted single cord blood transplantation. Biol Blood Marrow Transplant 22:1073–1079
Politikos I, Boussiotis VA (2014) The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood 124:3201–3211
Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, Brodin P (2018) Stereotypic immune system development in newborn children. Cell 174(1277-92):e14
Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK (2014) Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15:374–381
Huda MN, Ahmad SM, Alam MJ, Khanam A, Kalanetra KM, Taft DH, Raqib R, Underwood MA, Mills DA, Stephensen CB (2019) Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics 143:e20181489
Staffas A, Burgos da Silva M, Slingerland AE, Lazrak A, Bare CJ, Holman CD, Docampo MD, Shono Y, Durham B, Pickard AJ, Cross JR, Stein-Thoeringer C, Velardi E, Tsai JJ, Jahn L, Jay H, Lieberman S, Smith OM, Pamer EG, Peled JU, Cohen DE, Jenq RR, van den Brink MRM (2018) Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe 23(447-57):e4
Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, Ponce DM, Barker JN, Giralt S, van den Brink M, Pamer EG (2014) The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124:1174–1182
Gavriilaki M, Sakellari I, Anagnostopoulos A, Gavriilaki E (2020) The impact of antibiotic-mediated modification of the intestinal microbiome on outcomes of allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Biol Blood Marrow Transplant 26:1738–1746
Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC, Docampo MD, Peled JU, Arpaia N, Cross JR, Peets TK, Lumish MA, Shono Y, Dudakov JA, Poeck H, Hanash AM, Barker JN, Perales MA, Giralt SA, Pamer EG, van den Brink MR (2015) Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 21:1373–1383
Peled JU, Devlin SM, Staffas A, Lumish M, Khanin R, Littmann ER, Ling L, Kosuri S, Maloy M, Slingerland JB, Ahr KF, Porosnicu Rodriguez KA, Shono Y, Slingerland AE, Docampo MD, Sung AD, Weber D, Alousi AM, Gyurkocza B, Ponce DM, Barker JN, Perales MA, Giralt SA, Taur Y, Pamer EG, Jenq RR, van den Brink MRM (2017) Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol 35:1650–1659
Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, Weber D, Hashimoto D, Slingerland AE, Slingerland JB, Maloy M, Clurman AG, Stein-Thoeringer CK, Markey KA, Docampo MD, Burgos da Silva M, Khan N, Gessner A, Messina JA, Romero K, Lew MV, Bush A, Bohannon L, Brereton DG, Fontana E, Amoretti LA, Wright RJ, Armijo GK, Shono Y, Sanchez-Escamilla M, Castillo Flores N, Alarcon Tomas A, Lin RJ, Yanez San Segundo L, Shah GL, Cho C, Scordo M, Politikos I, Hayasaka K, Hasegawa Y, Gyurkocza B, Ponce DM, Barker JN, Perales MA, Giralt SA, Jenq RR, Teshima T, Chao NJ, Holler E, Xavier JB, Pamer EG, van den Brink MRM (2020) Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med 382:822–834
Schluter J, Peled JU, Taylor BP, Smith M, Markey KA, Taur Y, Niehus R, Staffas A, Dai A, Fontana E, Amoretti LA, Wright RJ, Morjaria S, Fenelus M, Pessin MS, Chao NJ, Lew M, Bohannon L, Bush A, Sung AD, Hohl TM, Perales M-A, van den Brink MRM, Xavier JB. 2020. An association between the gut microbiota and immune cell dynamics in humans. bioRxiv 618256
Vermijlen D, Prinz I (2014) Ontogeny of innate T lymphocytes - some innate lymphocytes are more innate than others. Front Immunol 5:486
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB (2015) The burgeoning family of unconventional T cells. Nat Immunol 16:1114–1123
Crosby CM, Kronenberg M (2018) Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol 18:559–574
Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D, Irving PM, John S, Mansour S, Bates PA, Vantourout P, Hayday AC (2018) The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol 19:1352–1365
Gnant M, Clezardin P (2012) Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev 38:407–415
Polito VA, Cristantielli R, Weber G, Del Bufalo F, Belardinilli T, Arnone CM, Petretto A, Antonucci L, Giorda E, Tumino N, Pitisci A, De Angelis B, Quintarelli C, Locatelli F, Caruana I (2019) Universal ready-to-use immunotherapeutic approach for the treatment of cancer: expanded and activated polyclonal gammadelta memory T cells. Front Immunol 10:2717
Arruda LCM, Gaballa A, Uhlin M (2019) Impact of gammadelta T cells on clinical outcome of hematopoietic stem cell transplantation: systematic review and meta-analysis. Blood Adv 3:3436–3448
Perko R, Kang G, Sunkara A, Leung W, Thomas PG, Dallas MH (2015) Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant 21:130–136
Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, Lamb LS (2007) Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant 39:751–757
Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, Meazza R, Loiacono F, Lucarelli B, Bernardo ME, Barbarito G, Pende D, Moretta A, Pistoia V, Moretta L, Locatelli F (2015) gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood 125:2349–2358
Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, Reinhardt A, Ravens I, Beck M, Geffers R, von Kaisenberg C, Heuser M, Thol F, Ganser A, Forster R, Koenecke C, Prinz I (2017) Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol 18:393–401
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP (2003) Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102:200–206
Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A (2003) Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 102:2310–2311
Bertaina A, Zorzoli A, Petretto A, Barbarito G, Inglese E, Merli P, Lavarello C, Brescia LP, De Angelis B, Tripodi G, Moretta L, Locatelli F, Airoldi I (2017) Zoledronic acid boosts gammadelta T-cell activity in children receiving alphabeta(+) T and CD19(+) cell-depleted grafts from an HLA-haplo-identical donor. Oncoimmunology 6:e1216291
Merli P, Algeri M, Galaverna F, Milano GM, Bertaina V, Biagini S, Girolami E, Palumbo G, Sinibaldi M, Becilli M, Leone G, Boccieri E, Grapulin L, Gaspari S, Airoldi I, Strocchio L, Pagliara D, Locatelli F (2020) Immune modulation properties of zoledronic acid on TcRgammadelta T-lymphocytes after TcRalphabeta/CD19-depleted haploidentical stem cell transplantation: an analysis on 46 pediatric patients affected by acute leukemia. Front Immunol 11:699
Rubio MT, Moreira-Teixeira L, Bachy E, Bouillie M, Milpied P, Coman T, Suarez F, Marcais A, Sibon D, Buzyn A, Caillat-Zucman S, Cavazzana-Calvo M, Varet B, Dy M, Hermine O, Leite-de-Moraes M (2012) Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival. Blood 120:2144–2154
Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E, McDonald D, Marin D, Milojkovic D, Pavlu J, Davis J, Rahemtulla A, Rezvani K, Goldman J, Roberts I, Apperley J, Karadimitris A (2012) Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood 119:5030–5036
Malard F, Labopin M, Chevallier P, Guillaume T, Duquesne A, Rialland F, Derenne S, Peterlin P, Leaute AG, Brissot E, Gregoire M, Moreau P, Saas P, Gaugler B, Mohty M (2016) Larger number of invariant natural killer T cells in PBSC allografts correlates with improved GVHD-free and progression-free survival. Blood 127:1828–1835
de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, Paganoni AM, Maccario R, Di Cesare-Merlone A, Zecca M, Locatelli F, Dellabona P, Casorati G (2011) Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state. J Immunol 186:4490–4499
Salou M, Legoux F, Gilet J, Darbois A, du Halgouet A, Alonso R, Richer W, Goubet AG, Daviaud C, Menger L, Procopio E, Premel V, Lantz O (2019) A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J Exp Med 216:133–151
Legoux F, Bellet D, Daviaud C, El Morr Y, Darbois A, Niort K, Procopio E, Salou M, Gilet J, Ryffel B, Balvay A, Foussier A, Sarkis M, El Marjou A, Schmidt F, Rabot S, Lantz O (2019) Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366:494–499
Varelias A, Bunting MD, Ormerod KL, Koyama M, Olver SD, Straube J, Kuns RD, Robb RJ, Henden AS, Cooper L, Lachner N, Gartlan KH, Lantz O, Kjer-Nielsen L, Mak JY, Fairlie DP, Clouston AD, McCluskey J, Rossjohn J, Lane SW, Hugenholtz P, Hill GR (2018) Recipient mucosal-associated invariant T cells control GVHD within the colon. J Clin Invest 128:1919–1936
van der Waart AB, van der Velden WJ, van Halteren AG, Leenders MJ, Feuth T, Blijlevens NM, van der Voort R, Dolstra H (2012) Decreased levels of circulating IL17-producing CD161+CCR6+ T cells are associated with graft-versus-host disease after allogeneic stem cell transplantation. PLoS One 7:e50896
Bhattacharyya A, Hanafi LA, Sheih A, Golob JL, Srinivasan S, Boeckh MJ, Pergam SA, Mahmood S, Baker KK, Gooley TA, Milano F, Fredricks DN, Riddell SR, Turtle CJ (2018) Graft-derived reconstitution of mucosal-associated invariant T cells after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 24:242–251
Ben Youssef G, Tourret M, Salou M, Ghazarian L, Houdouin V, Mondot S, Mburu Y, Lambert M, Azarnoush S, Diana JS, Virlouvet AL, Peuchmaur M, Schmitz T, Dalle JH, Lantz O, Biran V, Caillat-Zucman S (2018) Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J Exp Med 215:459–479
Konuma T, Kohara C, Watanabe E, Takahashi S, Ozawa G, Suzuki K, Mizukami M, Nagai E, Jimbo K, Kaito Y, Isobe M, Kato S, Takahashi S, Chiba A, Miyake S, Tojo A (2020) Reconstitution of circulating mucosal-associated invariant T cells after allogeneic hematopoietic Cell transplantation: its association with the riboflavin synthetic pathway of gut microbiota in cord blood transplant recipients. J Immunol 204:1462–1473
Park JE, Botting RA, Dominguez Conde C, Popescu DM, Lavaert M, Kunz DJ, Goh I, Stephenson E, Ragazzini R, Tuck E, Wilbrey-Clark A, Roberts K, Kedlian VR, Ferdinand JR, He X, Webb S, Maunder D, Vandamme N, Mahbubani KT, Polanski K, Mamanova L, Bolt L, Crossland D, de Rita F, Fuller A, Filby A, Reynolds G, Dixon D, Saeb-Parsy K, Lisgo S, Henderson D, Vento-Tormo R, Bayraktar OA, Barker RA, Meyer KB, Saeys Y, Bonfanti P, Behjati S, Clatworthy MR, Taghon T, Haniffa M, Teichmann SA (2020) A cell atlas of human thymic development defines T cell repertoire formation. Science 367:eaay3224
Verstichel G, Vermijlen D, Martens L, Goetgeluk G, Brouwer M, Thiault N, Van Caeneghem Y, De Munter S, Weening K, Bonte S, Leclercq G, Taghon T, Kerre T, Saeys Y, Van Dorpe J, Cheroutre H, Vandekerckhove B (2017) The checkpoint for agonist selection precedes conventional selection in human thymus. Sci Immunol 2:eaah4232
Ruscher R, Hogquist KA (2019) Development, ontogeny, and maintenance of TCRalphabeta(+) CD8alphaalpha IEL. Curr Opin Immunol 58:83–88
Ennamorati M, Vasudevan C, Clerkin K, Halvorsen S, Verma S, Ibrahim S, Prosper S, Porter C, Yeliseyev V, Kim M, Gardecki J, Sassi S, Tearney G, Cherayil BJ, Bry L, Seed B, Jain N (2020) Intestinal microbes influence development of thymic lymphocytes in early life. Proc Natl Acad Sci U S A 117:2570–2578
Calvo-Asensio I, Sugrue T, Bosco N, Rolink A, Ceredig R (2018) DN2 thymocytes activate a specific robust dna damage response to ionizing radiation-induced DNA double-strand breaks. Front Immunol 9:1312
Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR (2012) Interleukin-22 drives endogenous thymic regeneration in mice. Science 336:91–95
Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, Ottmuller KJ, Mokhtari Z, Brede C, deRoos P, Kinsella S, Palikuqi B, Ginsberg M, Young LF, Kreines F, Lieberman SR, Lazrak A, Guo P, Malard F, Smith OM, Shono Y, Jenq RR, Hanash AM, Nolan DJ, Butler JM, Beilhack A, Manley NR, Rafii S, Dudakov JA, van den Brink MRM (2018) Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Sci Immunol 3:eaal2736
Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL (2006) Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108:3777–3785
Calvo-Asensio I, Barthlott T, von Muenchow L, Lowndes NF, Ceredig R (2017) Differential response of mouse thymic epithelial cell types to ionizing radiation-induced DNA damage. Front Immunol 8:418
Sun DP, Jin H, Ding CY, Liang JH, Wang L, Fan L, Wu YJ, Xu W, Li JY (2016) Thymic hyperplasia after chemotherapy in adults with mature B cell lymphoma and its influence on thymic output and CD4(+) T cells repopulation. Oncoimmunology 5:e1137417
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, Wexler LH, Gress RE (1995) Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 332:143–149
Choyke PL, Zeman RK, Gootenberg JE, Greenberg JN, Hoffer F, Frank JA (1987) Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. AJR Am J Roentgenol 149:269–272
Velardi E, Dudakov JA, van den Brink MR (2015) Sex steroid ablation: an immunoregenerative strategy for immunocompromised patients. Bone Marrow Transplant 50(Suppl 2):S77–S81
Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373:1550–1561
Dertschnig S, Evans P, Santos ESP, Manzo T, Ferrer IR, Stauss HJ, Bennett CL, Chakraverty R (2020) Graft-versus-host disease reduces lymph node display of tissue-restricted self-antigens and promotes autoimmunity. J Clin Invest 130:1896–1911
Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, Carmagnat M, Rocha V, Charron D, Socie G, Toubert A (2009) Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood 113:6477–6484
Krenger W, Hollander GA (2008) The immunopathology of thymic GVHD. Semin Immunopathol 30:439–456
Gatza E, Reddy P, Choi SW (2020) Prevention and treatment of acute graft-versus-host disease in children, adolescents, and young adults. Biol Blood Marrow Transplant 26:e101–ee12
Kaebisch EM, Cho MY, Oh YS, Olfe LI, Szyska M, Becker SC, Reinke P, Volk HD, Gillissen B, Bullinger L, Thiel A, Na IK. 2019. Cytotoxic effects of rabbit anti-thymocyte globulin preparations on primary human thymic epithelial cells. Transplantation 103: 2234-44
Dertschnig S, Hauri-Hohl MM, Vollmer M, Hollander GA, Krenger W (2015) Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood 125:2720–2723
Kohler S, Thiel A (2009) Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood 113:769–774
Thome JJ, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, Sempowski GD, Shen Y, Farber DL (2016) Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol 1:eaah6506
Mold JE, Reu P, Olin A, Bernard S, Michaelsson J, Rane S, Yates A, Khosravi A, Salehpour M, Possnert G, Brodin P, Frisen J (2019) Cell generation dynamics underlying naive T-cell homeostasis in adult humans. PLoS Biol 17:e3000383
Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA (2000) Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 355:1875–1881
Ringhoffer S, Rojewski M, Dohner H, Bunjes D, Ringhoffer M (2013) T-cell reconstitution after allogeneic stem cell transplantation: assessment by measurement of the sjTREC/betaTREC ratio and thymic naive T cells. Haematologica 98:1600–1608
Hazenberg MD, Verschuren MC, Hamann D, Miedema F, Dongen JJ (2001) T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med 79:631–640
Torlen J, Gaballa A, Remberger M, Mork LM, Sundberg B, Mattsson J, Uhlin M (2019) Effect of graft-versus-host disease prophylaxis regimens on T and B cell reconstitution after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 25:1260–1268
Gaballa A, Sundin M, Stikvoort A, Abumaree M, Uzunel M, Sairafi D, Uhlin M (2016) T cell receptor excision circle (TREC) monitoring after allogeneic stem cell transplantation; a predictive marker for complications and clinical outcome. Int J Mol Sci 17:1705
Jung D, Alt FW (2004) Unraveling V(D)J Recombination: insights into gene regulation. Cell 116:299–311
Sewell AK (2012) Why must T cells be cross-reactive? Nat Rev Immunol 12:669–677
Alonso-Mora J, Samaranayake S, Wallar A, Frazzoli E, Rus D (2017) On-demand high-capacity ride-sharing via dynamic trip-vehicle assignment. Proc Natl Acad Sci U S A 114:462–467
Warren RL, Freeman JD, Zeng T, Choe G, Munro S, Moore R, Webb JR, Holt RA (2011) Exhaustive T-cell repertoire sequencing of human peripheral blood samples reveals signatures of antigen selection and a directly measured repertoire size of at least 1 million clonotypes. Genome Res 21:790–797
van Heijst JW, Ceberio I, Lipuma LB, Samilo DW, Wasilewski GD, Gonzales AM, Nieves JL, van den Brink MR, Perales MA, Pamer EG (2013) Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med 19:372–377
Meyer EH, Hsu AR, Liliental J, Lohr A, Florek M, Zehnder JL, Strober S, Lavori P, Miklos DB, Johnson DS, Negrin RS (2013) A distinct evolution of the T-cell repertoire categorizes treatment refractory gastrointestinal acute graft-versus-host disease. Blood 121:4955–4962
Yew PY, Alachkar H, Yamaguchi R, Kiyotani K, Fang H, Yap KL, Liu HT, Wickrema A, Artz A, van Besien K, Imoto S, Miyano S, Bishop MR, Stock W, Nakamura Y (2015) Quantitative characterization of T-cell repertoire in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 50:1227–1234
Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, Crawford JC, Clemens EB, Nguyen THO, Kedzierska K, La Gruta NL, Bradley P, Thomas PG (2017) Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547:89–93
Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, Ji X, Han A, Krams SM, Pettus C, Haas N, Arlehamn CSL, Sette A, Boyd SD, Scriba TJ, Martinez OM, Davis MM (2017) Identifying specificity groups in the T cell receptor repertoire. Nature 547:94–98
Schmitt TM, Zuniga-Pflucker JC (2002) Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17:749–756
Ohishi K, Varnum-Finney B, Bernstein ID (2002) Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J Clin Invest 110:1165–1174
Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR (2006) Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med 12:1039–1047
Zakrzewski JL, Suh D, Markley JC, Smith OM, King C, Goldberg GL, Jenq R, Holland AM, Grubin J, Cabrera-Perez J, Brentjens RJ, Lu SX, Rizzuto G, Sant’Angelo DB, Riviere I, Sadelain M, Heller G, Zuniga-Pflucker JC, Lu C, van den Brink MR (2008) Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol 26:453–461
Hoseini SS, Hapke M, Herbst J, Wedekind D, Baumann R, Heinz N, Schiedlmeier B, Vignali DA, van den Brink MR, Schambach A, Blazar BR, Sauer MG (2015) Inducible T-cell receptor expression in precursor T cells for leukemia control. Leukemia 29:1530–1542
Maluski M, Ghosh A, Herbst J, Scholl V, Baumann R, Huehn J, Geffers R, Meyer J, Maul H, Eiz-Vesper B, Krueger A, Schambach A, van den Brink MR, Sauer MG (2019) Chimeric antigen receptor-induced BCL11B suppression propagates NK-like cell development. J Clin Invest 129:5108–5122
Smith MJ, Reichenbach DK, Parker SL, Riddle MJ, Mitchell J, Osum KC, Mohtashami M, Stefanski HE, Fife BT, Bhandoola A, Hogquist KA, Holländer GA, Zúñiga-Pflücker JC, Tolar J, Blazar BR. (2017) T cell progenitor therapy–facilitated thymopoiesis depends upon thymic input and continued thymic microenvironment interaction JCI Insight 2:e92056
Awong G, Singh J, Mohtashami M, Malm M, La Motte-Mohs RN, Benveniste PM, Serra P, Herer E, van den Brink MR, Zuniga-Pflucker JC (2013) Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood 122:4210–4219
Reimann C, Six E, Dal-Cortivo L, Schiavo A, Appourchaux K, Lagresle-Peyrou C, de Chappedelaine C, Ternaux B, Coulombel L, Beldjord K, Cavazzana-Calvo M, Andre-Schmutz I (2012) Human T-lymphoid progenitors generated in a feeder-cell-free Delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/gammac(-/-) mice. Stem Cells 30:1771–1780
Simons L, Ma K, de Chappedelaine C, Moiranghtem RD, Elkaim E, Olivre J, Susini S, Appourchaux K, Reimann C, Sadek H, Pelle O, Cagnard N, Magrin E, Lagresle-Peyrou C, Taghon T, Rausell A, Cavazzana M, Andre-Schmutz I (2018) Generation of adult human T-cell progenitors for immunotherapeutic applications. J Allergy Clin Immunol 141:1491–4 e4
Shukla S, Langley MA, Singh J, Edgar JM, Mohtashami M, Zuniga-Pflucker JC, Zandstra PW (2017) Progenitor T-cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1. Nat Methods 14:531–538
Mackall CL, Fry TJ, Gress RE (2011) Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11:330–342
Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL (2008) Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 205:1701–1714
Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B, Rouzioux C, Avettand-Fenoel V, Croughs T, Beq S, Morre M, Poulin JF, Sekaly RP, Thiebaut R, Lederman MM (2012) Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis 55:291–300
Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL (2002) Interleukin 7 worsens graft-versus-host disease. Blood 100:2642–2649
Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, Young JW, Jakubowski AA, Zaidi B, Gallardo H, Liu C, Rasalan T, Wolchok JD, Croughs T, Morre M, Devlin SM, van den Brink MR (2012) Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood 120:4882–4891
Dudakov JA, Hanash AM, van den Brink MR (2015) Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33:747–785
Pan B, Liu J, Zhang Y, Sun Y, Wu Q, Zhao K, Zeng L, Xu K (2014) Acute ablation of DP thymocytes induces up-regulation of IL-22 and Foxn1 in TECs. Clin Immunol 150:101–108
Pan B, Zhang F, Lu Z, Li L, Shang L, Xia F, Fu R, Xu M, Zeng L, Xu K (2019) Donor T-cell-derived interleukin-22 promotes thymus regeneration and alleviates chronic graft-versus-host disease in murine allogeneic hematopoietic cell transplant. Int Immunopharmacol 67:194–201
Goldberg GL, Dudakov JA, Reiseger JJ, Seach N, Ueno T, Vlahos K, Hammett MV, Young LF, Heng TSP, Boyd RL, Chidgey AP (2010) Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J Immunol 184:6014–6024
Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL (2005) Effects of castration on thymocyte development in two different models of thymic involution. J Immunol 175:2982–2993
Williams KM, Lucas PJ, Bare CV, Wang J, Chu YW, Tayler E, Kapoor V, Gress RE (2008) CCL25 increases thymopoiesis after androgen withdrawal. Blood 112:3255–3263
Velardi E, Tsai JJ, Holland AM, Wertheimer T, Yu VW, Zakrzewski JL, Tuckett AZ, Singer NV, West ML, Smith OM, Young LF, Kreines FM, Levy ER, Boyd RL, Scadden DT, Dudakov JA, van den Brink MR (2014) Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J Exp Med 211:2341–2349
Khong DM, Dudakov JA, Hammett MV, Jurblum MI, Khong SM, Goldberg GL, Ueno T, Spyroglou L, Young LF, van den Brink MR, Boyd RL, Chidgey AP (2015) Enhanced Hematopoietic Stem Cell Function Mediates Immune Regeneration following Sex Steroid Blockade. In: Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Reports 4:445–458
Dudakov JA, Goldberg GL, Reiseger JJ, Chidgey AP, Boyd RL (2009) Withdrawal of sex steroids reverses age- and chemotherapy-related defects in bone marrow lymphopoiesis. J Immunol 182:6247–6260
Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL (2005) Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 175:2741–2753
Sutherland JS, Spyroglou L, Muirhead JL, Heng TS, Prieto-Hinojosa A, Prince HM, Chidgey AP, Schwarer AP, Boyd RL (2008) Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res 14:1138–1149
Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI (2002) Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood 99:4592–4600
Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, Suh D, Muriglan SJ, Boyd RL, van den Brink MR (2006) Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood 107:2453–2460
Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, Blazar BR (2008) Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood 111:5734–5744
Blazar BR, Weisdorf DJ, Defor T, Goldman A, Braun T, Silver S, Ferrara JL (2006) Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood 108:3216–3222
Stiff PJ, Leinonen M, Kullenberg T, Rudebeck M, de Chateau M, Spielberger R (2016) Long-term safety outcomes in patients with hematological malignancies undergoing autologous hematopoietic stem cell transplantation treated with palifermin to prevent oral mucositis. Biol Blood Marrow Transplant 22:164–169
Lopes N, Vachon H, Marie J, Irla M (2017) Administration of RANKL boosts thymic regeneration upon bone marrow transplantation. EMBO Mol Med 9:835–851
Zlotoff DA, Zhang SL, De Obaldia ME, Hess PR, Todd SP, Logan TD, Bhandoola A (2011) Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood 118:1962–1970
Hess E, Duheron V, Decossas M, Lezot F, Berdal A, Chea S, Golub R, Bosisio MR, Bridal SL, Choi Y, Yagita H, Mueller CG (2012) RANKL induces organized lymph node growth by stromal cell proliferation. J Immunol 188:1245–1254
Yun HD, Waller EK (2013) Finding the sweet spot for donor lymphocyte infusions. Biol Blood Marrow Transplant 19:507–508
Marktel S, Magnani Z, Ciceri F, Cazzaniga S, Riddell SR, Traversari C, Bordignon C, Bonini C (2003) Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood 101:1290–1298
Tiberghien P, Ferrand C, Lioure B, Milpied N, Angonin R, Deconinck E, Certoux JM, Robinet E, Saas P, Petracca B, Juttner C, Reynolds CW, Longo DL, Herve P, Cahn JY (2001) Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood 97:63–72
Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C (1997) HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276:1719–1724
Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, Turchetto L, Colombi S, Bernardi M, Peccatori J, Pescarollo A, Servida P, Magnani Z, Perna SK, Valtolina V, Crippa F, Callegaro L, Spoldi E, Crocchiolo R, Fleischhauer K, Ponzoni M, Vago L, Rossini S, Santoro A, Todisco E, Apperley J, Olavarria E, Slavin S, Weissinger EM, Ganser A, Stadler M, Yannaki E, Fassas A, Anagnostopoulos A, Bregni M, Stampino CG, Bruzzi P, Bordignon C (2009) Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol 10:489–500
Vago L, Oliveira G, Bondanza A, Noviello M, Soldati C, Ghio D, Brigida I, Greco R, Lupo Stanghellini MT, Peccatori J, Fracchia S, Del Fiacco M, Traversari C, Aiuti A, Del Maschio A, Bordignon C, Ciceri F, Bonini C (2012) T-cell suicide gene therapy prompts thymic renewal in adults after hematopoietic stem cell transplantation. Blood 120:1820–1830
Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM (2005) An inducible caspase 9 safety switch for T-cell therapy. Blood 105:4247–4254
Zhang P, Raju J, Ullah MA, Au R, Varelias A, Gartlan KH, Olver SD, Samson LD, Sturgeon E, Zomerdijk N, Avery J, Gargett T, Brown MP, Coin LJ, Ganesamoorthy D, Hutchins C, Pratt GR, Kennedy GA, Morton AJ, Curley CI, Hill GR, Tey SK (2019) Phase I trial of inducible caspase 9 T cells in adult stem cell transplant demonstrates massive clonotypic proliferative potential and long-term persistence of transgenic T cells. Clin Cancer Res 25:1749–1755
Zhou X, Di Stasi A, Tey SK, Krance RA, Martinez C, Leung KS, Durett AG, Wu MF, Liu H, Leen AM, Savoldo B, Lin YF, Grilley BJ, Gee AP, Spencer DM, Rooney CM, Heslop HE, Brenner MK, Dotti G (2014) Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood 123:3895–3905
Brodin P, Davis MM (2017) Human immune system variation. Nat Rev Immunol 17:21–29
Lakshmikanth T, Olin A, Chen Y, Mikes J, Fredlund E, Remberger M, Omazic B, Brodin P (2017) Mass cytometry and topological data analysis reveal immune parameters associated with complications after allogeneic stem cell transplantation. Cell Rep 20:2238–2250
Stikvoort A, Chen Y, Radestad E, Torlen J, Lakshmikanth T, Bjorklund A, Mikes J, Achour A, Gertow J, Sundberg B, Remberger M, Sundin M, Mattsson J, Brodin P, Uhlin M (2017) Combining flow and mass cytometry in the search for biomarkers in chronic graft-versus-host disease. Front Immunol 8:717
Patin E, Hasan M, Bergstedt J, Rouilly V, Libri V, Urrutia A, Alanio C, Scepanovic P, Hammer C, Jonsson F, Beitz B, Quach H, Lim YW, Hunkapiller J, Zepeda M, Green C, Piasecka B, Leloup C, Rogge L, Huetz F, Peguillet I, Lantz O, Fontes M, Santo JP, Thomas S, Fellay J, Duffy D, Quintana-Murci L, Albert ML, Milieu IC (2018) Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol 19:302–314
Clave E, Araujo IL, Alanio C, Patin E, Bergstedt J, Urrutia A, Lopez-Lastra S, Li Y, Charbit B, MacPherson CR, Hasan M, Melo-Lima BL, Douay C, Saut N, Germain M, Tregouet DA, Morange PE, Fontes M, Duffy D, Di Santo JP, Quintana-Murci L, Albert ML, Toubert A, Milieu IC (2018) Human thymopoiesis is influenced by a common genetic variant within the TCRA-TCRD locus. Sci Transl Med:10:eaao2966
Kim S, Shah SB, Graney PL, Singh A (2019) Multiscale engineering of immune cells and lymphoid organs. Nat Rev Mater 4:355–378
Hun M, Barsanti M, Wong K, Ramshaw J, Werkmeister J, Chidgey AP (2017) Native thymic extracellular matrix improves in vivo thymic organoid T cell output, and drives in vitro thymic epithelial cell differentiation. Biomaterials 118:1–15
Fan Y, Tajima A, Goh SK, Geng X, Gualtierotti G, Grupillo M, Coppola A, Bertera S, Rudert WA, Banerjee I, Bottino R, Trucco M (2015) Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol Ther 23:1262–1277
Bortolomai I, Sandri M, Draghici E, Fontana E, Campodoni E, Marcovecchio GE, Ferrua F, Perani L, Spinelli A, Canu T, Catucci M, Di Tomaso T, Sergi Sergi L, Esposito A, Lombardo A, Naldini L, Tampieri A, Hollander GA, Villa A, Bosticardo M (2019) Gene modification and three-dimensional scaffolds as novel tools to allow the use of postnatal thymic epithelial cells for thymus regeneration approaches. Stem Cells Transl Med 8:1107–1122
Poznansky MC, Evans RH, Foxall RB, Olszak IT, Piascik AH, Hartman KE, Brander C, Meyer TH, Pykett MJ, Chabner KT, Kalams SA, Rosenzweig M, Scadden DT (2000) Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat Biotechnol 18:729–734
Seet CS, He C, Bethune MT, Li S, Chick B, Gschweng EH, Zhu Y, Kim K, Kohn DB, Baltimore D, Crooks GM, Montel-Hagen A (2017) Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat Methods 14:521–530
Shah NJ, Mao AS, Shih T-Y, Kerr MD, Sharda A, Raimondo TM, Weaver JC, Vrbanac VD, Deruaz M, Tager AM, Mooney DJ, Scadden DT (2019) An injectable bone marrow–like scaffold enhances T cell immunity after hematopoietic stem cell transplantation. Nat Biotechnol 37:293–302
Montel-Hagen A, Seet CS, Li S, Chick B, Zhu Y, Chang P, Tsai S, Sun V, Lopez S, Chen HC, He C, Chin CJ, Casero D, Crooks GM (2019) Organoid-induced differentiation of conventional T cells from human pluripotent stem cells. Cell Stem Cell 24:376–89 e8
Ulyanchenko S, O’Neill KE, Medley T, Farley AM, Vaidya HJ, Cook AM, Blair NF, Blackburn CC (2016) Identification of a bipotent epithelial progenitor population in the adult thymus. Cell Rep 14:2819–2832
Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, Boehm T (2006) Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature 441:992–996
Sun X, Xu J, Lu H, Liu W, Miao Z, Sui X, Liu H, Su L, Du W, He Q, Chen F, Shi Y, Deng H (2013) Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell 13:230–236
Parent AV, Russ HA, Khan IS, Laflam TN, Metzger TC, Anderson MS, Hebrok M (2013) Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell 13:219–229
Inami Y, Yoshikai T, Ito S, Nishio N, Suzuki H, Sakurai H, Isobe K (2011) Differentiation of induced pluripotent stem cells to thymic epithelial cells by phenotype. Immunol Cell Biol 89:314–321
Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC (2014) An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat Cell Biol 16:902–908
Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM, Batchvarova MN, Douek DC, Koup RA, Kostyu DD, Ward FE, Rice HE, Mahaffey SM, Schiff SE, Buckley RH, Haynes BF (1999) Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med 341:1180–1189
Funding
E.V. was supported by grants from the Amy Strelzer Manasevit Research Program; the Italian Association for Cancer Research (AIRC); and the Italian Ministry of Health (“Ricerca Corrente”). F.L. was supported by grants from AIRC (Special Program Metastatic disease: the key unmet need in oncology 5 per mille 2018 Project Code 21147 and Accelerator Award 2017 INCAR); Ministero dell’Istruzione, dell’Università e della Ricerca, PRIN ID 2017 WC8499_004; Ministero della Salute, RF-2016-02364388. A.T. and E.C. were supported by the French Government’s Investissement d’Avenir Program, Laboratoire d’Excellence “Milieu Intérieur” Grant ANR-10-LABX-69-01 and by the by the Agence Nationale de la Recherche (Project RANKLthym ANR-19- CE18-0021-02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.V. has acted as a consultant and received honorarium from Ferring Pharmaceuticals. Two provisional patent applications have been filed (US 15/033,178 and US 62/566,897) with E.V. listed as inventor. F.L. participated in advisory boards or speakers bureaus for Amgen, Novartis, Bellicum Pharmaceutical, Miltenyi, Medac, Neovii, Jazz Pharmaceutical and Takeda. A.T., F.B., and E.C. have no conflict of interest.
Additional information
This article is a contribution to the special issue on: The thymus and autoimmunity - Guest Editor: Georg Holländer.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Velardi, E., Clave, E., Arruda, L.C.M. et al. The role of the thymus in allogeneic bone marrow transplantation and the recovery of the peripheral T-cell compartment. Semin Immunopathol 43, 101–117 (2021). https://doi.org/10.1007/s00281-020-00828-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-020-00828-7