Abstract
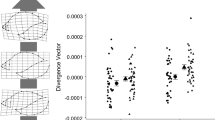
Phenotypic plasticity is common among animal taxa. While there are clearly limits and likely costs to plasticity, these costs are unknown for most organisms. Further, as plasticity is partially genetically determined, the potential magnitude of exhibited plasticity may vary among individuals. In addition to phenotypic plasticity, various animal taxa also display sexual size dimorphism, a feature ultimately thought to arise due to differential size-dependent fitness costs and benefits between sexes. We hypothesized that differential selection acting on males and females can indirectly select for unequal genetically defined plasticity potential between the sexes. We evaluate this possibility for Eurasian perch (Perca fluviatilis), a species that displays modest sexual size dimorphism and habitat-related morphological plasticity. Using 500-year simulations of an ecogenetic agent-based model, we demonstrate that genetically determined morphological plasticity potential may evolve differently for males and females, leading to greater realized morphological variation between habitats for one sex over the other. Genetically determined potential for plasticity evolved differently between sexes across (a) various sex-specific life-history differences and (b) a variety of assumed costs of plasticity acting on both growth and survival. Morphological analyses of Eurasian perch collected in situ were consistent with model predictions: realized morphological variation between habitats was greater for females than males. We suggest that due to sex-specific selective pressures, differences in male and female genetically defined potential for plasticity may be a common feature across organisms.




Similar content being viewed by others
Data availability
Upon acceptance of paper for publication, the baseline model code will be archived within the Purdue University Research Repository (https://purr.purdue.edu/).
References
Adams DC, Otárola-Castillo E (2013) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399
Agrawal AA, Conner JK, Johnson MTJ, Wallsgrove R (2002) Ecological genetics of an induced plant defense against herbivores: additive genetic variance and costs of phenotypic plasticity. Evolution 56:2206–2213
Andree SR, Feiner ZS, Bledsoe JW, Cragun AM, Höök TO (2015) Ontogenetic variability of maternal effects in an iteroparous fish. Ecol Freshw Fish 24:384–396
Auld JR, Agrawal AA, Relyea RA (2010) Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proceedings of the Royal Society B 277:503–511
Bartels P, Hirsch P, Svanbäck R, Eklöv P (2012) Water transparency drives intra-population divergence in Eurasian perch (Perca fluviatilis). PLoS ONE 7:e43641
Blanckenhorn WU, Preziosi RF, Fairbairn DJ (1995) Time and energy constraints and the evolution of sexual size dimorphism—to eat or to mate? Evol Ecol 9:369–381
Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, Svanbäck R (2014) Individual diet has sex-dependent effects on vertebrate gut microbiota. Nature Communications. https://doi.org/10.1038/ncomms5500
Dall SRX, Bell AM, Ratnieks BDIFW (2012) An evolutionary ecology of individual differences. Ecol Lett 15:1189–1198
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dryden IL (2015). shapes package. R Foundation for Statistical Computing, Vienna, Austria. Contributed package. Version 1.1–11. URL http://www.R-project.org
Dunlop ES, Shuter BJ, Ridgway MS (2005) Isolating the influence of growth rate on maturation patterns in the smallmouth bass (Micropterus dolomieu). Can J Fish Aquat Sci 62:844–853
Dunlop ES, Heino M, Dieckmann U (2009) Eco-genetic modeling of contemporary life-history evolution. Ecol Appl 19:1815–1834
Eklöv P, Svanbäck R (2006) Predation risk influences adaptive morphological variation in fish populations. Am Nat 1673:440–452
Fairbairn DJ, Blanckenhorn WU, Székely T (2007) Sex, size, and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford
Faulks L, Svanbäck R, Eklöv P, Östman Ö (2015) Genetic and morphological divergence along the littoral–pelagic axis in two common and sympatric fishes: perch, Perca fluviatilis (Percidae) and roach, Rutilus rutilus (Cyprinidae). Biol J Lin Soc 114:929–940
Feiner ZS, Höök TO (2015) Environmental biology of percids. in biology and culture of percid fishes—principles and practices. Kestemont P, Dabrowski K, and Summerfelt RC (Eds.)
Feiner ZS, Chong SC, Knight CT, Lauer TE, Thomas MV, Tyson JT, Höök TO (2015) Rapidly shifting maturation schedules following reduced commercial harvest in a freshwater fish. Evol Appl 8:724–737
Feiner ZS, Chong SC, Fielder DG, Hoyle JA, Knight C, Lauer TE, Thomas MV, Tyson JT, Höök TO (2017) Sex-based tradeoffs between growth, mortality, and maturation in Great Lakes yellow perch stocks. Can J Fish Aquat Sci 74:2059–2072
Gross MR (1984) Sunfish, salmon and the evolution of alternative reproductive strategies and tactics in fishes. In: Potts GW, Wooten RJ (eds) Fish reproduction: strategies and tactics. Academic Press, London, pp 55–73
Heibo E, Magnhagen C, Vøllestad LA (2005) Latitudinal variation in life-history traits in Eurasian perch. Ecology 86:3377–3386
Henderson BA, Wong JL, Nepszy SJ (1996) Reproduction of walleye in Lake Erie: allocation of energy. Can J Fish Aquat Sci 53:127–133
Henderson BA, Collins N, Morgan GE, Vaillancourt A (2003) Sexual size dimorphism of walleye (Stizostedion vutreum vitreum). Can J Fish Aquat Sci 60:1345–1352
Hendry AP (2016) Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J Hered 107:25–41
Herczeg G, Gonda A, Balázs G, Noreikiene K, Merilä J (2015) Experimental evidence for sex-specific plasticity in adult brain. Front Zool 12:38
Hirsch P, Eklöv P, Svanbäck R (2013) Indirect trophic interactions with an invasive species affect phenotypic divergence in a top consumer. Oecologia 172:245–256
Hjelm J, Svanbäck R, Byström P, Persson L, Wahlström E (2001) Diet-dependent body morphology and ontogenetic reaction norms in Eurasian perch. Oikos 95:311–323
Ivan LN, Höök TO (2015) Energy allocation strategies of young temperate fish: an eco-genetic modeling approach. Can J Fish Aquat Sci 72:1243–1258
Legrand RS, Morse DH (2000) Factors driving extreme sexual size dimorphism of a sit-and-wait predator under low density. Biol J Lin Soc 71:642–664
Marklund MHK, Svanbäck R, Zha Y, Scharnweber K, Eklöv P (2018) The influence of habitat accessibility on the dietary and morphological specialisation of an aquatic predator. Oikos 127:160–169
Meuthen D, Baldauf SA, Baker TCM, Thünken T (2018) Neglected patterns of variation in phenotypic plasticity: age and sex-specific antipredator plasticity in a cichlid fish. Am Nat 191:475–490
Murren CJ, Auld JR, Callahan H, Ghalambor CK, Handelsman CA, Heskel MA, Schlichting CD (2015) Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115:292–301
Nonaka E, Brännström A, Svanbäck R (2014) Assortative mating can limit the evolution of phenotypic plasticity. Evol Ecol 28:1057–1074
Olin M, Jutila J, Lehtonen H, Vinni M, Ruuhijarvi J, Estlander S, Lappalainen J (2012) Importance of maternal size on the reproductive success of perch, Perca fluviatilis, in small forest lakes: implications for fisheries management. Fish Manage Ecol 19:363–374
Olsson J, Eklöv P (2005) Habitat structure, feeding mode and morphological reversibility: factors influencing phenotypic plasticity in perch. Evol Ecol Res 7:1109–1123
Olsson J, Svanbäck R, Eklöv P (2006) Growth rate constrain morphological divergence when driven by competition. Oikos 115:15–22
Olsson J, Svanbäck R, Eklöv P (2007) Effects of resource level and habitat type on behavioural and morphological plasticity in Eurasian perch. Oecologia 152:48–56
Parker GA (1992) The evolution of sexual size dimorphism in fish. J Fish Biol 41:1–20
Price TD (2006) Phenotypic plasticity, sexual selection and the evolution of colour patterns. J Experiment Biol 209:2368–2376
Quevedo M, Svanbäck R, Eklöv P (2009) Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 90:2263–2274
Rohde K, Dreher E, Hochkirch A (2015) Sex-specific phenotypic plasticity in response to the trade-off between development time and body size supports the dimorphic niche hypothesis. Biol J Linnean Soc 115:48–57
Rueffler C, Egas M, Metz JA (2006) Evolutionary predictions should be based on individual-level traits. Am Nat 168:E148–E162
Scheffer M, Baveco MJ, DeAngelis DL, Rose KA, van Nes EH (1995) Super-individuals a simple solution for modeling large population on an individual basis. Ecol Model 80:161–170
Skúlason S, Smith TB (1995) Resource polymorphisms in vertebrates. Trends Ecol Evol 10:366–370
Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW (2010) Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Entomology 55:227–245
Svanbäck R, Eklöv P (2002) Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 131:61–70
Svanbäck R, Eklöv P (2003) Morphology dependent foraging efficiency in perch: a trade-off for ecological specialization? Oikos 102:273–284
Svanbäck R, Eklöv P (2004) Morphology in perch affects habitat specific feeding efficiency. Funct Ecol 18:503–510
Svanbäck R, Eklöv P (2006) Genetic variation and phenotypic plasticity: causes of morphological variation in Eurasian perch. Evol Ecol Res 8:37–49
Svanbäck R, Johansson F (2019) Predation selects for smaller eye size in a vertebrate: effects of environmental conditions and sex. Proc Royal Soc B 286:20182625
Svanbäck R, Persson L (2004) Individual diet specialization, niche width and population dynamics: implications for trophic polymorphisms. J Anim Ecol 73:973–982
Svanbäck R, Persson L (2009) Population density fluctuations change the selection gradient in Eurasian perch. Am Nat 173:507–516
Svanbäck R, Eklöv P, Fransson R, Holmgren K (2008) Intraspecific competition drives multiple species resource polymorphism in fish communities. Oikos 117:114–124
Van Buskirk J, Steiner UK (2009) The fitness costs of developmental canalization and plasticity. J Evol Biol 22:852–860
Wang HY, Höök TO (2009) Eco-genetic model to explore fishing-induced ecological and evolutionary effects on growth and maturation schedules. Evol Appl 2:438–455
Zelditch M, Swiderski D, Sheets H, Fink W (2004) Geometric morphometrics for biologists. A primer. Elselvier Academic Press, San Francisco
Funding
TH developed the model described herein while on sabbatical from Purdue University and visiting Uppsala University. Funding was provided by Purdue University and subsidized by support from the American-Scandinavian Foundation.
Author information
Authors and Affiliations
Contributions
TH, RS and PE conceived the study and designed the model and analyses; TH developed the ecogenetic model code and analyzed the model output; RS compiled and analyzed the empirical data; TH led the writing of the manuscript. All the authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All research presented in the manuscript was conducted in accordance with all applicable laws and rules set forth by governments and institutions and all necessary permits were acquired when the research was conducted.
Additional information
Communicated by Donald DeAngelis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Höök, T.O., Svanbäck, R. & Eklöv, P. Sex-specific plasticity in a trophic polymorphic aquatic predator: a modeling approach. Oecologia 195, 341–354 (2021). https://doi.org/10.1007/s00442-020-04843-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04843-1