Abstract
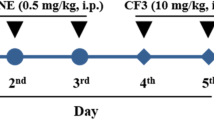
Energy-dense foods and ethanol consumption are associated with mood disorders. m-Trifluoromethyl-diphenyl diselenide [(m-CF3-PhSe)2] has been a prominent pharmacological target due to its antidepressant-like effects. This study investigated if the modulation of opioid and glucocorticoid receptors and its well-known antioxidant property contribute to the (m-CF3-PhSe)2 antidepressant-like effect in young mice subjected to an energy-dense diet and ethanol intake. Swiss male mice [postnatal day (PND) 25] were exposed to an energy-dense diet (containing 20% fat and 20% carbohydrate) or standard chow until the PND 67. Mice received ethanol (2 g/kg) or water administration (3 times a week, intragastrically [i.g.]) from PND 45 to PND 60. After that, mice received (m-CF3-PhSe)2 (5 mg/kg/day; i.g) or vegetal oil administration from PND 60 to 66. Mice performed the behavioral tests to evaluate the depressive-like phenotype. The results showed that individually neither an energy-dense diet nor ethanol group induced a depressive-like phenotype, but the association of both induced this phenotype in young mice. Oxidative stress was characterized by the increase of malondialdehyde, the decrease in the superoxide dismutase activity, and non-protein sulfhydryl levels in the cerebral cortex of depressive-like mice. Depressive-like mice showed an increase in the protein levels of opioid receptors and depletion in those of glucocorticoid. (m-CF3-PhSe)2 abolished depressive-like phenotype and oxidative stress as well as modulated the levels of glucocorticoid and opioid receptors. In conclusion, the modulation of opioid and glucocorticoid receptors and the antioxidant property contributed to the (m-CF3-PhSe)2 antidepressant-like effect in young mice exposed to an energy-dense diet and ethanol intake.





Similar content being viewed by others
Data Availability
Not applicable.
References
Will MJ, Franzblau EB, Kelley AE (2003) Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23(7):2882–2888
Mai BH, Yan LJ (2019) The negative and detrimental effects of high fructose on the liver, with special reference to metabolic disorders. Diabetes Metab Syndr Obes 12:821–826. https://doi.org/10.2147/DMSO.S198968
Gomes JAS, Silva JF, Marcal AP, Silva GC, Gomes GF, de Oliveira ACP, Soares VL, Oliveira MC et al (2020) High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice. J Nutr Biochem 77:108317. https://doi.org/10.1016/j.jnutbio.2019.108317
Del Rio D, Morales L, Ruiz-Gayo M, Del Olmo N (2016) Effect of high-fat diets on mood and learning performance in adolescent mice. Behav Brain Res 311:167–172. https://doi.org/10.1016/j.bbr.2016.04.052
Blanco-Gandia MC, Ledesma JC, Aracil-Fernandez A, Navarrete F, Montagud-Romero S, Aguilar MA, Manzanares J, Minarro J et al (2017) The rewarding effects of ethanol are modulated by binge eating of a high-fat diet during adolescence. Neuropharmacology 121:219–230. https://doi.org/10.1016/j.neuropharm.2017.04.040
Gass JT, Glen WB Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB et al (2014) Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39(11):2570–2583. https://doi.org/10.1038/npp.2014.109
Chastain G (2006) Alcohol, neurotransmitter systems, and behavior. J Gen Psychol 133(4):329–335
Schweitzer P, Cates-Gatto C, Varodayan FP, Nadav T, Roberto M, Lasek AW, Roberts AJ (2016) Dependence-induced ethanol drinking and GABA neurotransmission are altered in Alk deficient mice. Neuropharmacology 107:1–8
Rada P, Avena NM, Hoebel BG (2005) Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134(3):737–744. https://doi.org/10.1016/j.neuroscience.2005.04.043
Valdivia S, Cornejo MP, Reynaldo M, De Francesco PN, Perello M (2015) Escalation in high fat intake in a binge eating model differentially engages dopamine neurons of the ventral tegmental area and requires ghrelin signaling. Psychoneuroendocrinology 60:206–216. https://doi.org/10.1016/j.psyneuen.2015.06.018
Organization WH (2017) Depression and other common mental disorders: Global health estimates. World Health Organization
Dean J, Keshavan M (2017) The neurobiology of depression: an integrated view. Asian J Psychiatr 27:101–111. https://doi.org/10.1016/j.ajp.2017.01.025
Dubinina EE, Morozova MG, Leonova NV, Gamper NL, Soliternova IB, Nuller Iu L, Butoma GB, Kovrugina SV (2000) Oxidative modification of blood proteins in patients with psychiatric disorders (depression, depersonalization). Vopr Med Khim 46(4):398–409
Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, Meaney MJ, Nestler EJ (2018) Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev 84:272–288. https://doi.org/10.1016/j.neubiorev.2017.08.019
Nogueira CW, Rocha JB (2011) Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Arch Toxicol 85(11):1313–1359. https://doi.org/10.1007/s00204-011-0720-3
Segat H, Martini F, Barcelos R, Brüning C, Nogueira C, Burger M (2016) m-Trifluoromethyl-diphenyldiselenide as a pharmacological tool to treat preference symptoms related to AMPH-induced dependence in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 66:1–7
Rosa SG, Pesarico AP, Martini F, Nogueira CW (2018) M-Trifluoromethyl-diphenyl diselenide regulates prefrontal cortical MOR and KOR protein levels and abolishes the phenotype induced by repeated forced swim stress in mice. Mol Neurobiol 55(12):8991–9000. https://doi.org/10.1007/s12035-018-1024-x
Lu C, Sun T, Li Y, Zhang D, Zhou J, Su X (2017) Modulation of the gut microbiota by krill oil in mice fed a high-sugar high-fat diet. Front Microbiol 8:905. https://doi.org/10.3389/fmicb.2017.00905
Matheus VA, Monteiro L, Oliveira RB, Maschio DA, Collares-Buzato CB (2017) Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp Biol Med (Maywood) 242(12):1214–1226. https://doi.org/10.1177/1535370217708188
Paulmier C (1986) Selenium reagents and intermediates. Organic Synthesis Pergamon, Oxford
Duly AM, Alani B, Huang EY, Yee C, Haber PS, McLennan SV, Seth D (2015) Effect of multiple binge alcohol on diet-induced liver injury in a mouse model of obesity. Nutr Diabetes 5:e154. https://doi.org/10.1038/nutd.2015.4
Sanchez-Roige S, Pena-Oliver Y, Ripley TL, Stephens DN (2014) Repeated ethanol exposure during early and late adolescence: Double dissociation of effects on waiting and choice impulsivity. Alcohol Clin Exp Res 38(10):2579–2589. https://doi.org/10.1111/acer.12535
Rosa SG, Pesarico AP, Tagliapietra CF, da Luz SC, Nogueira CW (2017) Opioid system contribution to the antidepressant-like action of m-trifluoromethyl-diphenyl diselenide in mice: a compound devoid of tolerance and withdrawal syndrome. J Psychopharmacol 31(9):1250–1262. https://doi.org/10.1177/0269881117724353
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370. https://doi.org/10.1007/bf00428203
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266(5604):730–732. https://doi.org/10.1038/266730a0
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Uytun MC (2018) Development Period of Prefrontal Cortex. In: Development period of prefrontal cortex. Prefrontal Cortex. IntechOpen, In
Locateli G, de Oliveira Alves B, Miorando D, Ernetti J, Alievi K, Zilli GAL, Serpa PZ, Vecchia CAD, Mota da Silva L, Muller LG, Roman Junior WA (2020) Antidepressant-like effects of solidagenone on mice with bacterial lipopolysaccharide (LPS)-induced depression. Behav Brain Res:112863. doi:https://doi.org/10.1016/j.bbr.2020.112863
Mayur P, Murat A, Malone DA Jr, Anand A (2012) Where in the brain is depression? Current Psychiatry Reports 14(6):634–642
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TGM et al (2017) Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatry 22(6):900–909. https://doi.org/10.1038/mp.2016.60
Cong X, Tracy M, Edmunds LS, Hosler AS, Appleton AA (2019) The relationship between inflammatory dietary pattern in childhood and depression in early adulthood. Brain, Behavior, & Immunity-Health:100017
Penasco S, Rico-Barrio I, Puente N, Fontaine CJ, Ramos A, Reguero L, Gerrikagoitia I, de Fonseca FR et al (2020) Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor-dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 45(2):309–318. https://doi.org/10.1038/s41386-019-0530-5
Kuria MW, Ndetei DM, Obot IS, Khasakhala LI, Bagaka BM, Mbugua MN, Kamau J (2012) The association between alcohol dependence and depression before and after treatment for alcohol dependence. ISRN Psychiatry 2012:482802–482806. https://doi.org/10.5402/2012/482802
Hassan AM, Mancano G, Kashofer K, Frohlich EE, Matak A, Mayerhofer R, Reichmann F, Olivares M et al (2019) High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr Neurosci 22(12):877–893. https://doi.org/10.1080/1028415X.2018.1465713
Yuan F, Lei Y, Wang Q, Esberg LB, Huang Z, Scott GI, Li X, Ren J (2015) Moderate ethanol administration accentuates cardiomyocyte contractile dysfunction and mitochondrial injury in high fat diet-induced obesity. Toxicol Lett 233(3):267–277. https://doi.org/10.1016/j.toxlet.2014.12.018
Alwahsh SM, Xu M, Schultze FC, Wilting J, Mihm S, Raddatz D, Ramadori G (2014) Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS One 9(8):e104220. https://doi.org/10.1371/journal.pone.0104220
Quines CB, Rosa SG, Chagas PM, Velasquez D, Prado VC, Nogueira CW (2017) (p-ClPhSe)2 stimulates carbohydrate metabolism and reverses the metabolic alterations induced by high fructose load in rats. Food Chem Toxicol 107 (Pt A):122-128. doi:https://doi.org/10.1016/j.fct.2017.06.038
Muller SG, Jardim NS, Quines CB, Nogueira CW (2018) Diphenyl diselenide regulates Nrf2/Keap-1 signaling pathway and counteracts hepatic oxidative stress induced by bisphenol A in male mice. Environ Res 164:280–287. https://doi.org/10.1016/j.envres.2018.03.006
Bae JY, Shin KO, Woo J, Woo SH, Jang KS, Lee YH, Kang S (2016) Exercise and dietary change ameliorate high fat diet induced obesity and insulin resistance via mTOR signaling pathway. J Exerc Nutr Biochem 20(2):28–33
Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J, Zhou GY, Fang CW et al (2020) Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol 36:101635. https://doi.org/10.1016/j.redox.2020.101635
Alwahsh SM, Gebhardt R (2017) Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch Toxicol 91(4):1545–1563. https://doi.org/10.1007/s00204-016-1892-7
Beccaria G (2017) Depressive disorders. Abnormal psychology in context: the Australian and New Zealand handbook:94
Nogueira CW, Zeni G, Rocha JB (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 104(12):6255–6285. https://doi.org/10.1021/cr0406559
Rosa SG, Pesarico AP, Nogueira CW (2018) m-Trifluoromethyl-diphenyl diselenide promotes resilience to social avoidance induced by social defeat stress in mice: Contribution of opioid receptors and MAPKs. Prog Neuro-Psychopharmacol Biol Psychiatry 82:123–135. https://doi.org/10.1016/j.pnpbp.2017.11.021
Nogueira C, Meotti F, Curte E, Pilissao C, Zeni G, Rocha J (2003) Investigations into the potential neurotoxicity induced by diselenides in mice and rats. Toxicology 183(1–3):29–37
Bruning CA, Prigol M, Roehrs JA, Nogueira CW, Zeni G (2009) Involvement of the serotonergic system in the anxiolytic-like effect caused by m-trifluoromethyl-diphenyl diselenide in mice. Behav Brain Res 205(2):511–517. https://doi.org/10.1016/j.bbr.2009.08.010
Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr, Jones RM, Portoghese PS et al (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305(1):323–330. https://doi.org/10.1124/jpet.102.046433
Le AD, Poulos CX, Quan B, Chow S (1993) The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res 630(1–2):330–332. https://doi.org/10.1016/0006-8993(93)90672-a
Hall FS, Sora I, Uhl GR (2001) Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology 154(1):43–49. https://doi.org/10.1007/s002130000622
Lutz PE, Kieffer BL (2013) Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36(3):195–206. https://doi.org/10.1016/j.tins.2012.11.002
Madden CJ (2018) Kappa opioid receptor activation in the preoptic area (POA) contributes to the impairment of brown adipose tissue activation in rats on a high fat diet (HFD). The FASEB Journal 32 (1_supplement):592.596–592.596
Zhang M, Gosnell BA, Kelley AE (1998) Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285(2):908–914
Nogueiras R, Romero-Pico A, Vazquez MJ, Novelle MG, Lopez M, Dieguez C (2012) The opioid system and food intake: homeostatic and hedonic mechanisms. Obes Facts 5(2):196–207. https://doi.org/10.1159/000338163
Berg D, Youdim MB, Riederer P (2004) Redox imbalance. Cell Tissue Res 318(1):201–213. https://doi.org/10.1007/s00441-004-0976-5
Raut A, Rao VR, Ratka A (2007) Changes in opioid receptor proteins during mitochondrial impairment in differentiated SK-N-SH cells. Neurosci Lett 422(3):187–192. https://doi.org/10.1016/j.neulet.2007.06.015
Czarny P, Wigner P, Galecki P, Sliwinski T (2018) The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry 80 (Pt C):309-321. doi:https://doi.org/10.1016/j.pnpbp.2017.06.036
Farrell C, O'Keane V (2016) Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Res 242:349–356. https://doi.org/10.1016/j.psychres.2016.06.022
Code Availability
Not applicable.
Funding
We gratefully acknowledge Universidade Federal de Santa Maria (UFSM). This study was funded by Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS, grant number 17/2551-0000), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 407118/2018-7), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX #23038.004173/2019-93). C.W.N is recipient of CNPq fellowship (#304864/2015-3). N.S.J (#88882.182156/2018-01) and S.G.M. (#88887.372342/2019-00) are recipient of CAPES fellowships.
Author information
Authors and Affiliations
Contributions
S.G.M, N.S.J, and C.W.N. conceived and designed the study. S.G.M, N.S.J, and M.A.T. conducted behavioral tests and ex vivo and molecular analyses. S.G.M, N.S.J, and C.W.N. wrote and reviewed the manuscript. C.W.N. supervised the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Maria, Brazil (Permit Number:7141061018), and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All efforts were made to reduce the number and suffering of animals to minimum.
Conflict of Interest
The authors declare they have no conflicts of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 82 kb)
Rights and permissions
About this article
Cite this article
Müller, S.G., Jardim, N.S., Trindade, M.A. et al. Opioid System Contributes to the Trifluoromethyl-Substituted Diselenide Effectiveness in a Lifestyle-Induced Depression Mouse Model. Mol Neurobiol 58, 2231–2241 (2021). https://doi.org/10.1007/s12035-020-02255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02255-z