Abstract
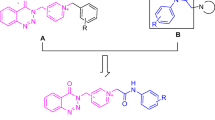
A Series of new tacrine analogs were designed, synthesized, characterized by respective spectral data and evaluated for cholinesterase inhibitory activity to be useful in Alzheimer’s disease. Most of the synthesized compounds showed good in vitro inhibitory activities toward acetyl cholinesterase (AChE) and butyrylcholinesterase (BuChE) enzymes. Among the compounds, 6i, 6o and 6r with increased saturated carboxylic ring size attached to the pyridine moiety and having 3,4-dihydroxy, 3,4,5-trimethoxy substituents on the aromatic ring attached at the stereogenic center have shown equal potency to that of tacrine with IC50values 0.65 ± 0.06, 1.32 ± 0.02 and 0.85 ± 0.05, 1.65 ± 0.12 and 0.92 ± 0.03, 1.91 ± 0.12 μM against AChE and BuChE, respectively. Standard drug tacrine exhibited IC50 values of 0.47 ± 0.02 and 0.65 ± 0.08, while Donepezil showed IC50 0.71 ± 0.06 and 0.31 ± 0.04 μM against AChE and BuChE, respectively. Docking studies of all the molecules disclosed close hydrogen bond interactions with the binding site.












Similar content being viewed by others
References
Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–81. https://doi.org/10.1126/science.1132814
Wimo A, Prince M, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015. 2015. http://www.alz.co.uk/worldreport
Andreeva T, Lukiw WJ, Rogaev EI. Biological basis for Amyloidogenesis in Alzheimer’s disease. Biochem. (Moscow). 2017;82:122–39. https://doi.org/10.1134/s0006297917020043
Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer’s disease and other neurodegenerative disorders. Annal Neuro. 2011;70:532–40. https://doi.org/10.1002/ana.22615
Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimer’s Dis. 2010;19:341–53. https://doi.org/10.3233/jad-2010-1222
Kaur R, Mehan S, Singh S. Understanding multifactorial architecture of Parkinson’s disease: pathophysiology to management. Neurological Sci. 2019;40:13–23. https://doi.org/10.1007/s10072-018-3585-x
Meng Q, Ru J, Zhang GL. Re-evaluation of tacrine hepatotoxicity using gel entrapped hepatocytes. Toxicol Lett. 2007;168:140–7. https://doi.org/10.1016/j.toxlet.2006.11.009
Wu WY, Chendai YU, Gu T. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem. 2017;32:572–87. https://doi.org/10.1080/14756366.2016.1210139
Cavalli A, Bolognesi ML, Minarini A. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–72. https://doi.org/10.1021/jm7009364
Chen X, Decker ME. Multi-target compounds acting in the central nervous system designed from natural products. Cur Med Chem. 2013;20:1673–85. https://doi.org/10.2174/0929867311320130007
Wang Y, Wang F, Yu JP. Novel multipotent phenylthiazole-tacrine hybrids for the inhibition of cholinesterase activity, β-Amyloid aggregation and Ca²+ overload. Bioorg Med Chem. 2012;20:6513–522. https://doi.org/10.1016/j.bmc.2012.08.040
Mohamed T, Rao PP. Alzheimer’s disease: emerging trends in small molecule therapies. Cur Med Chem. 2011;18:4299–320. https://doi.org/10.2174/092986711797200435
Eghtedari M, Sarrafi Y, Nadri H, Mahdavi M, Moradi A, Homayouni MF, et al. New tacrine-derived AChE/BuChE inhibitors: Synthesis and biological evaluation of 5-amino-2-phenyl-4H-pyrano[2,3-b]quinoline-3-carboxylates. Eur J Med Chem. 2017;128:237–46. https://doi.org/10.1016/j.ejmech.2017.01.042
Pourabdi L, Khoobi M, Nadri H, Moradi A, Moghadam F, Emami S, et al. Synthesis and structure activity relationship study of tacrine-based pyrano[2,3-c] pyrazole stargeting AChE/BuChE and 15-LOX. Eur J Med Chem. 2016;123:298–308. https://doi.org/10.1016/j.ejmech.2016.07.043
Maalej E, Chabchoub F, Oset-Gasque MJ, Samadi MJ. Synthesis, biological assessment, and molecular modeling of racemic 7-aryl-9,10,11,12-tetrahydro-7H-benzo [7,8] chromeno[2,3-b]quinolin-8-amines as potential drugs for the treatment of Alzheimer’s disease. Eur J MedChem. 2012;54:750–63. https://doi.org/10.1016/j.ejmech.2012.06.038
Dgachi Y, Bautista OM, Benchekroun M, Martin H, Bonet A, Marco-Contelles J, et al. Synthesis and biological evaluation of benzochromenopyrimidinones as cholinesterase inhibitors and potent antioxidant, non-hepatotoxic agents for Alzheimer’s disease. Mol. 2016;21:634. https://doi.org/10.3390/molecules21050634
Hanish Singh JC, Alagarasamy V, Diwan PV, Kumar SS, Nisha JS, Reddy YN. Neuroprotective effect of Alpiniagalanga (L) fractions on Aβ(25-35) induced amnesia in mice. J Ethno Pharmacol. 2011;138:85–91. https://doi.org/10.1016/j.jep.2011.08.048
Almoazen H, Bhattacharjee H, Samse AC, Patel S. Stability of mesna in readymade infusion devices. Ann Pharmacother. 2010;44:224–5. https://doi.org/10.1345/2Faph.1M390
Ellman GL, Courtney KD, Anders V, Featherstone RM. New and rapid colorimetric determination of acetyl cholinesterase activity. Biochem Pharmacol. 1961;7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Barros DM, Izquierdo LA, Mello ST, Ardenghi P, Pereira P, Medina JH, et al. Molecular signaling pathways in the cerebral cortex are required for retrieval of one‐trial inhibitory avoidance learning in rats. Behav Brain Res. 2000;114:183–92. https://doi.org/10.1016/S0166-4328(00)00226-6
Bornschein RL, Hastings L, Manson JM. Behavioral toxicity in the offspring of rats following maternal exposure to dichloromethane. Toxic Appl Pharmacol. 1980;52:29–37. https://doi.org/10.1016/0041-008X(80)90244-6
Garofalo P, Colombo S, Lanza M, Revel L, Makovec F. New putative memory enhancer, behavioral studies on learning and memory in rats and mice. J Pharm Pharmacol. 1996;48:1290–7. https://doi.org/10.1111/j.2042-7158.1996.tb03938.x
Buresova O, Bures J, Oitzl MSA. Radial maze in the water tank: an aversively motivated spatial working memory task Zahalka. Physiol Behav. 1985;34:1003–5. https://doi.org/10.1016/0031-9384(85)90028-9
Shear DA, Dong J, Creguer KL, Bazzett TJ, Albin RL, Dunbar GL. Chronic administration of quinolinic acid in the rat striatum causes spatial learning deficits in a radial arm water maze task. Exp Neurol. 1998;150:305–11. https://doi.org/10.1006/exnr.1998.6767
Goodarzi Z, Mele B, Guo S, Hanson H, Jette N, Patten S, et al. Guidelines for dementia or Parkinson’s disease with depression or anxiety, a systematic review. BMC Neurol. 2016;16:244–8. https://doi.org/10.1186/s12883-016-0754-5
Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS. The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res. 1997;759:76–83. https://doi.org/10.1016/S0006-8993(97)00236-9
Letteron P, Labbe G, Degott C, Berson A, Fromenty B, Larrey M, et al. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice, evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Bio Chem Pharmacol. 1990;39:2027–34. https://doi.org/10.1016/0006-2952(90)90625-u
Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY, Shyu JC, et al. Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. J Viral Hep. 2008;15:508–14. https://doi.org/10.1111/j.1365-2893.2008.00971x
Schrödinger Release 2019-1: Schrödinger Suite 2019-1 Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2019
Dizdaroglu Y, Albay C, Arslan T, Ece A, Emir A, Turkoglu E, et al. Design, synthesis and molecular modelling studies of some pyrazole derivatives as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2020;35:289–97. https://doi.org/10.1080/14756366.2019.1695791
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26:1701–18. https://doi.org/10.1002/jcc.20291
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comp Chem. 2010;31:671–90. https://doi.org/10.1002/jcc.21367
Vanommeslaeghe K, MacKerell AD Jr. Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J Chem Inf Model. 2012;52:3144–54. https://doi.org/10.1021/ci300363c
Bjelkmar P, Larsson P, Cuendet MA, Hess B, Lindahl E. Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. J Chem Theory Comp. 2010;6:459–66. https://doi.org/10.1021/ct900549r
Acknowledgements
One of the authors BM expresses thank to the University Grants Commission, New Delhi for financial support of this study. Authors thank the Principal, University College of Pharmaceutical Sciences, Kakatiya University, Warangal for providing necessary facilities. Authors RK and CB express gratitude Mr. Raghu Rangaswami and Dr. Prajwal Nandekar, Schrodinger, Bangalore for granting free one-monthlicense of Schrodinger Suite 2019 for docking studies. Both RK and CB appreciate the Principal and Management of Bharati Vidyapeeth’s Poona College of Pharmacy, Pune for providing all computational facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Macha, B., Kulkarni, R., Bagul, C. et al. Molecular hybridization based design and synthesis of new benzo[5,6]chromeno[2,3-b]-quinolin-13(14H)-one analogs as cholinesterase inhibitors. Med Chem Res 30, 685–701 (2021). https://doi.org/10.1007/s00044-020-02670-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02670-w