Abstract
Communication about threats including those posed by the presence of predators occurs mainly through acoustic signals called alarm calls. The comprehension of these calls by receivers and their rapid antipredator response are crucial in terms of survival. However, to avoid overreaction, individuals should evaluate whether or not an antipredator response is needed by paying attention to who is calling. For instance, we could expect adults to be more experienced with predator encounters than juveniles and thus elicit stronger antipredator responses in others when alarming. Similarly, we could expect a stronger response to alarm calls when more than one individual is calling. To test these assumptions, we applied a playback experiment to wild ravens, in which we manipulated the age class (adult or juvenile) and the number (one or two) of the callers. Our results revealed a seasonal effect of age class but no effect of number of callers. Specifically, the ravens responded with stronger antipredator behaviour (vigilance posture) towards alarm calls from adults as compared to juveniles in summer and autumn, but not in spring. We discuss alternative interpretations for this unexpected seasonal pattern and argue for more studies on call-based communication in birds to understand what type of information is relevant under which conditions.
Similar content being viewed by others
Introduction
Birds are famous for their vocalization. Song learning, for instance, has been intensively studied over the last decades (Catchpole & Slater, 2008) and is fairly well understood from a behavioural and neurobiological perspective (Bolhuis & Gahr, 2006), making it an excellent model for human speech (Bolhuis, Okanoya, & Scharff, 2010). In comparison to the vast literature on song learning, bird calls have received limited attention (Marler, 2004), and studies on the cognitive skills underlying the production and usage of bird calls are rare. In respect to the latter, research on a single grey parrot, ‘Alex’, has become famous: using English words for communicating with human trainers, Alex not only labelled objects, but responded to questions probing his knowledge (e.g. of relational concepts like same/different) and expressed intentions via requests (Pepperberg, 1999). While Alex’s skills are impressive in many ways, sparking debates on various conceptual and methodological levels (Pepperberg, 1983, 1990, 2008), his apparent understanding and intentional use of communicative signals with humans raises the question of what predispositions these skills might be based upon? Why would a grey parrot like Alex need a sophisticated neuro-cognitive machinery allowing him to copy sounds, attach meaning to it, form concepts, and use them in interaction with others? Twenty-five years of research on grey parrots´ life support the idea of evolutionary pressures underpinning complex communicative and cognitive capacities (Pepperberg, 2002). It has been argued that parrots need such abilities in daily social life (Pepperberg, 1999), which in the case of Alex, happened to be the human setting. But what challenges could parrots, or other birds, face under ecologically relevant situations that require communication other than song, i.e. that they should ‘talk’ about?
Obvious candidates are live-threatening events, like predator encounters, that can occur to wild animals at any time. Using communication may help individuals to detect predators (Smith, 1965; Zuberbühler, Noë, & Seyfarth, 1997) and assess the type or level of threat (Seyfarth, Cheney, & Marler, 1980), and, as a consequence, respond with appropriate behaviours such as escaping, hiding or repealing an attack (Botham et al., 2008; Kotler, Blaustein, & Brown, 1992; Lohrey, Clark, Gordon, & Uetz, 2009). Potential victims may also gather forces and drive the predator away from the area (Foster & Treherne, 1981). While acoustic signals given in the presence of predators are commonly referred to as alarm calls (Hauser, 1996), the behaviour associated with driving the predator away is known as mobbing or collective anti-predator behaviour (Curio, 1978; Graw & Manser, 2007).
Like many mammals, birds tend to give different alarm calls to specific events in the environment, like the occurrence of ground or areal predators (Evans, Evans, & Marler, 1993). Avian alarm calls are thus a prime candidate for investigating information content about external reference (Gill & Bierema, 2013). Experiments revealed that in some species, alarm calls denote different types of predator classes that require different response strategies (Kalb, Anger, & Randler, 2019; Suzuki, 2012, 2014), which fulfil the criteria of functional reference (Evans et al., 1993; Rendall, Owren, & Ryan, 2009); in other species, however, the calls denote the urgency level to respond (Leavesley & Magrath, 2005), and thus likely represent differences in arousal states (Blumstein & Récapet, 2009). Typically, alarm calls have a strong genetic component in respect to production, but are relatively flexible in respect to usage (Fichtel & Van Schaik, 2006; Townsend, Rasmussen, Clutton-Brock, & Manser, 2012). Senders may thus fine-tune the use of alarm calls, for example to denote a specific predator type or behaviour (Griesser, 2008; Suzuki, Wheatcroft, & Griesser, 2016), and/or adjust their signalling to the audience, for example call more when kin or mating partners are present (e.g. Shields, 1984). On the receiver side, getting accurate information about predators and learning to respond appropriately to alarm calls are of high survival value (Griesser, 2013). Receivers may readily learn about alarm calls even across species, as demonstrated in the mobbing flocks of mixed-species communities of songbirds (Magrath, Pitcher, & Gardner, 2009; Wheatcroft, Gallego-Abenza, & Qvarnström, 2016).
Like most vocalizations, alarm calls also contain information about the sender, such as its sex, age class, kin or individual identity (Blumstein & Munos, 2005). Receivers of alarm calls may thus not only respond to the type of threat/level of urgency encoded in the calls but take the senders’ features and/or identities into account when engaging in antipredator behaviour (Hare, 1998; Hare & Atkins, 2001). Surprisingly few studies have tested the receivers’ responses to such sender-specific characteristics in birds (with the exception of kin in nepotistic alarm calling and/or mobbing, e.g. Griesser & Ekman, 2004, 2005). Experiments on Pied flycatchers (Ficedula hypoleuca) showed that they do not automatically respond to any alarm calls of their territory neighbours with predator mobbing but selectively help those neighbours to mob a predator, who had helped them before (Krams, Krama, Igaune, & Mänd, 2008; Wheatcroft & Price, 2008). The reciprocal pattern indicates that the birds acquire some form of knowledge and/or attribute about their neighbours through previous predator encounters. Recent experiments on jackdaws (Corvus monedula) revealed that birds respond stronger with collective anti-predator behaviour to the play back alarm calls of colony members as compared to non-colony members, indicating that receivers discriminate between familiar and unfamiliar birds (Woods, Kings, McIvor, & Thornton, 2018). Furthermore, the number of callers had a similar positive effect on the probability to engage in collective antipredator behaviour, indicating that receivers take into account whether the alarm calls were elicited by a single or a few birds and hence the intensity of the response (Coomes, Mcivor, Thornton, Coomes, & Thornton, 2019). Such assessment capability by receivers was also documented in small mammals, precisely in adult Richardson´s ground squirrels (Sloan & Hare, 2008). When confronted with alarm calls from conspecifics and closely related heterospecifics during foraging, carrion crows tended to respond to any alarm calls (Bílá, Beránková, Veselý, Bugnyar, & Schwab, 2017), whereas ravens adjusted their antipredator behaviour depending on the perceived risk (whether or not they snatched food from predators; Nácarová, Veselý, & Bugnyar, 2018) and the familiarity of the calling species (Davidkova, Veselý, Syrova, Nacarovà, & Bugnyar, 2020).
In the present study, we followed the logic of the studies on jackdaws (Coomes et al., 2019) and investigated whether common ravens are attentive to sender-specific characteristics in alarm calls. Unlike jackdaws, adult ravens defend large territories (Scarpignato & George, 2011) and thus do not form colonies during breeding. However, non-breeding ravens tend to form large groups, usually near food sources (Heinrich, 1989; Loretto, Schuster, & Bugnyar, 2016). These groups are composed mainly of immature individuals (juveniles in their first year, subadults in their second or third year; making up about 20% and 60% of a group, respectively) but also of adult birds (older than 3 years, often having no partner and/or no territory; typically about 20% of a group) (Braun & Bugnyar, 2012; Heinrich, 1989). Apart from age-class, raven foraging groups are structured by social relationships (social bonds based on reciprocal exchange of affiliative interactions; Braun & Bugnyar 2012) and kinship (Szipl, Ringler, & Bugnyar, 2018; but see Parker, Waite, Heinrich, & Marzluff, 1994). Yet, the foraging groups have an open character, with individuals coming and going on a regular basis (Heinrich, 1989). How long birds stay at a site/in a given group varies extensively from a few days to years; hence, individuals can meet rarely, regularly or frequently at the same or different sites (Loretto et al., 2017). Taken together, the socially structured but fluid nature of raven foraging groups constitutes a promising scenario for studying what individuals know about others and which features they attend to (Boucherie, Loretto, Massen, & Bugnyar, 2019). With respect to alarm calls, we could expect adults to be more reliable in terms of threat perception than young individuals, due to the adults’ previous life-experience encountering different types of threats. Furthermore, we could expect not only the number of callers but also the identity of the callers to be critical for the receivers’ decision to engage in antipredator behaviour.
Here we focussed on two of the identified factors, age class (as a proxy for experience) and number and callers (as a proxy for threat intensity), while controlling for the callers’ identity (always unfamiliar). We exposed groups of free-ranging ravens during foraging to playbacks of a standardized number of alarm calls given either by a single juvenile, two juveniles, a single adult or two adults. We predicted ravens to show stronger responses when listening to adult individuals as compared to juveniles and when listening to two different individuals alarming as compared to one individual.
Material and methods
Study site and study species
This study was conducted at the Cumberland Wildpark, a zoo in the Austrian Alps (N 47°48.421′, E 13°57.032′), where groups of common ravens snatch food from animal enclosures. These ravens are the focus of a long-term monitoring program (started in 2007), during which more than 300 birds have been marked with rings and wing tags for individual identification. The size of the daily foraging groups in the park ranges between 20 and 80 individuals. The groups are composed mostly of non-breeders in the first years of life (juveniles and subadults, < 4 years old) but also adults that do not hold a territory and/or visit this group in the non-breeding period opportunistically; they continuously change in composition with noticeable individual differences in terms of how long ravens stay and/or leave (Braun & Bugnyar, 2012; Loretto et al., 2017). We focused on the wild boar enclosure for our experiment, as this enclosure allows a good view of the foraging ravens, the wild boars themselves do not represent a risk for the ravens, and the ravens are known for responding well to playbacks of heterospecific and conspecific calls at this location (Nácarová et al., 2018). A total of 48 trials were conducted in three different seasons, starting in spring 2019 (17 January 2019–7 May 2019), followed by autumn (25 September 2019–18 December 2019) and finishing in summer 2020 (25 May 2020–18 July 2020), with two non-testing periods of approximately 4 and 7 months between seasons.
Playback stimuli
We used alarm calls that were recorded from captive ravens at Haidlhof Research Station, which is located in the east of Austria, about 200 km away from our field site in the Alps. While our marked wild ravens may roam over larger areas (Loretto et al., 2017), they have never been observed near Haidlhof.
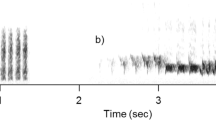
Ravens at Haidlhof were housed in a social group structured by age class (juveniles, subadults and adults) simulating the wild conditions. In an experimental study, these ravens were exposed to a human carrying a dead raven resulting in intense mobbing behaviour and alarm calling (Blum, Fitch, & Bugnyar, 2020). We used these calls from that experiment because: (1) these captive ravens were unfamiliar to the wild ravens in Grünau, and (2) we could identify callers at the individual level. The known identity of callers allowed us to compose the four different treatments: single caller versus two callers from either juvenile or adult age class, thus creating 16 different broadcasting files (four per each treatment) to be broadcast in randomized order within and among each season, conducting 16 trials per season, 48 trials in total. Testing days were separated from each other by 4.3 days on average (range 2–18) to avoid habituation. Sex was also known and controlled within a treatment composition, generating a similar number of broadcasting files of each sex. Each treatment was composed using four calls, where the third and fourth calls occurred after 3 s of silence interval and could correspond to either the same or a different individual (see Fig. 1). We equalized all calls´ amplitude in the composed files to be broadcast using Audacity software (https://www.audacityteam.org/). Alarm calls were played back in .wav format using a digital music player (Musrun k188) connected to a loudspeaker (JBL xtreme, frequency response 70–20,000 Hz). All calls were standardized to an identical volume of 73 dB measured at 2 m of distance (Sound Level Meter RadioShack, model 3300099, A-weighting, fast response).
Behavioural responses
Playbacks were conducted during the feedings of wild boars, i.e. while the wild ravens were foraging. The same experimenter (MGA) conducted all trials to avoid a potential effect of experimenter identity (MGA has been studying the ravens at this location for than 2 years: hence, the ravens were well habituated to his presence from the beginning of the experiment). Ravens´ responses were filmed using two GoPro Hero 5 cameras from fixed positions at 2 m and 5 m of distance to the foraging site. We measured the total number of ravens present in the camera´s field of view. In addition, we scored whether ravens in the video were flying off from the foraging place or adopted a vigilance posture for 5 s right after broadcasting the alarm calls. We defined vigilance posture as being when ravens raised up their heads, elongating their necks, and directed their gaze repeatedly towards sky, while switching between eyes, following studies on antipredator behaviours conducted in other avian species (Fernández-Juricic, 2012; Guillemain, Duncan, & Fritz, 2001).
Statistical analyses
We conducted our statistical analyses in R software (v. 3.6.1., R Development Core Team 2014). For modelling the two response variables: `vigilance posture´ and `flying off´, we used the function glmer in the package `lme4´ (Bates, Mächler, Bolker, & Walker, 2015). Due to an inconstant number of total ravens across testing days, we used the command `cbind´ within the model formula to control for it, thus modelling the proportion of ravens showing any of the two behavioural responses (`vigilance posture´ and `flying off´) with a binomial error distribution. To answer the question whether ravens responded differently depending on the `treatment´ (single adult, two adults, single juvenile or two juveniles) being exposed to, we used `season´ as random factor, together with `broadcasting file´, controlling for potential seasonal effects. When testing the effect of `season´ on ravens´ response, we included it as fixed factor and ´broadcasting file´ remained as unique random factor. When using `age class´ or `calling composition´ (one or two callers), trials were clumped together according to these predictors. Model selection through both AICc and weight comparison was conducted using the function `model.sel´, ´MuMIn´ package (Barton, 2019). The best model explaining `vigilance posture´ response contained the interaction between `age class´ and `season´, in order to examine significant differences within each season, we conducted a post hoc Tukey contrast test using `emmeans´ package (Searle, Speed, & Milliken, 1980) to calculate differences in estimated marginal means and P values.
Results
Ravens responded to played back alarm calls by adopting `vigilance posture´, in 46 out of 48 trials (95.8%), whereas a `flying off´ response occurred in only 12 out of 48 trials (25%). For both behavioural response variables, the model containing `treatment´ as unique explanatory predictor did not result in a significant difference between the four playback conditions (single adult, two adults, single juvenile, two juveniles). However, model selection indicated that for `vigilance posture´ the model containing the interaction between `season´ and `age class´ was the best model (lower AICc and higher weight; see Tables 1 and 2). `Age class´ independently affected ravens´ vigilance response, where ravens were less responsive to juvenile compared to adult callers (Estimate = -0.777, SE = 0.342, Z = -2.272, P = 0.023). Similarly, we found that `season´ had an effect on the ravens´ vigilance posture response to any played-back alarm call´s composition, whereby higher vigilance posture values occurred in summer (Estimate = 0.563, SE = 0.259, Z = 2.172, P = 0.029). Additionally, the interaction between the two abovementioned factors revealed age-specific responses depending on the season. The post hoc Tukey contrast test revealed that stronger responses to adult as compared to juvenile callers occurred in summer and autumn, but not in spring (Fig. 2).
A similar procedure with model selection was followed to estimate how `season´ and `age class´ affected the `flying off´ response. In this case, the model containing the interaction between `season´ and `treatment´ was classified as the best model (Supplementary Table 1). However, neither of those had a significant effect (separately or in interaction) on ´flying off´ response (Supplementary Table 2).
Discussion
We tested whether wild ravens respond to two types of information possibly encoded in conspecifics alarm calls, i.e. the age class of the caller, and whether calls are given by one or more individuals. Playbacks of alarm calls elicited a stronger vigilance response when given by adults as compared to juveniles in two out of three seasons (in summer and autumn, not in spring). The number of calling individuals, however, did not lead to a significant difference in the ravens´ antipredator responses.
That ravens respond more strongly to alarm calls of adults rather than juveniles meets our expectation and supports the assumption that receivers can extract information about the age class of alarm callers. That this effect is dependent on season was not expected, however, and may be explained in different ways. On the one hand, ravens might have responded less to alarm calls given by juveniles in summer and autumn because at that time juveniles are very young and likely lack experience with predators and/or may easily give alarm calls to any disturbing situation. Hence, juveniles might be perceived as less reliable in alarm calling than adults. Similar findings have been described in some studies on mammals (Ramakrishnan & Coss, 2000; Seyfarth & Cheney, 1986), whereas other studies reported no effect of age class (Swan & Hare, 2008) or even the opposite (Blumstein & Daniel, 2004). On the other hand, ravens might be particularly receptive to alarm calls in spring, when local low temperatures allow them to scavenge on carcasses, putting them into increased contact with predators, and their survival rates are lowest (Webb, Boarman, & Rotenberry, 2004). Hence, in the season with a high likelihood of dangerous predator encounters, they might respond to any alarm calls, irrespective of the caller´s age class. Similar patterns of seasonal variation in antipredator behaviour have been described for other species (Shedd, 1982; Uchida, Suzuki, Shimamoto, Yanagawa, & Koizumi, 2016). Interestingly, the temporal pattern of our results renders either interpretation unlikely (Fig. 2). Receivers did not increase their response to juvenile alarm calls across the year, as would be expected with increasing experience of young birds or with increased threat levels after the first winter; conversely, they decreased their response to alarm calls of adults across the seasons, showing the lowest response rates in spring. This pattern suggests that ravens treat alarm calls of adults particularly seriously during summer and autumn, i.e. the period when families with young ravens are around. Alternatively, the pattern could be interpreted as resulting from reduced attention towards alarm calls of adults during cold periods (winter-spring). Although ravens face severe foraging competition in winter (Heinrich, 1989) and may divide their attention between gaining access to food (B. Heinrich & Marzluff, 1995), fending off conspecific kleptoparasitism and cache pilferage (Bugnyar & Kotrschal, 2002; Gallego-Abenza, Loretto, & Bugnyar, 2020; Heinrich & Pepper, 1998), there are hardly any indications that competition for food affects their antipredator behaviour, at least not at our study site (Nácarová et al., 2018). Finally, the temporal pattern found might be considered an artefact of our testing. Note that the order of playback presentation (first in spring 2019, then in autumn 2019, and then in summer 2020) makes it unlikely that our results are due to an order effect or habituation. Moreover, the played-back individuals were unfamiliar to the tested ravens, indicating that receivers can extract age-class information from any conspecific alarm calls, which is perfectly in line with the ecological relevance of alarm calls (Gill & Bierema, 2013) and the structure of raven foraging groups with moderate to high fission-fusion dynamics (Braun, Walsdorff, Fraser, & Bugnyar, 2012).
Contrary to our expectation and to recent findings in jackdaws (Coomes et al., 2019), we could not find any effect of the number of played-back individuals on ravens’ antipredator behaviour. Our failure to detect a numerical discrimination through alarm calls may be due to the salience of the chosen stimuli. For instance, while we used one or two callers, the study on jackdaws used one, three or five callers; it is known that animals, including birds, have more difficulties in discriminating one versus two in comparison to one versus larger numbers (Tornick, Callahan, & Gibson, 2015). In a study conducted on mammals, more precisely on Richardson´s Ground Squirrels, only adult receivers showed enhanced antipredator responsiveness to two versus one alarm caller, even though juvenile receivers discriminate among individual callers, suggesting a developmental shift in the parameters employed to assess the veracity of any threat (Sloan & Hare, 2008). Alternatively, the ravens might have a problem in picking up on the individual information in the calls. We already know that some ravens’ calls like food-associated calls (`haa´) and territory calls (`rab´) contain strong individual signatures, which the birds respond to in habituation-dishabituation experiments (Boeckle, Szipl, & Bugnyar, 2012); in other calls, like those given in agonistic interactions, individual information is less evident in comparison to affective information (Szipl, Ringler, Spreafico, & Bugnyar, 2017). Possibly, this is similar with alarm calls. A proper acoustical analysis and further playback experiments are needed to investigate this question.
Taken together, our study contributes to our understanding of what type of information birds may pick up when hearing alarm calls. While most studies on alarm calls have focused on functional reference about predators (Evans et al., 1993; Griesser, 2008; Suzuki, 2011, 2014), relatively few studies have experimentally tested for other types of information, like familiarity of caller/group membership (Griesser & Ekman, 2004, 2005; Woods et al., 2018), number of callers and callers´ age class (Coomes et al., 2019; this study). The findings reveal that birds respond selectively to different features that appear to be ecologically relevant, like the seasonal effect of responding to adults in this study. What is yet unknown is how much birds make use of individual information encoded in alarm calls, as several results could be explained by (refined) class-level discrimination (Tibbetts & Dale, 2007). In this respect, studies on behavioural deception are interesting, as there are multiple reports of individual callers becoming unreliable when repeatedly giving false alarms (Flower, Gribble, & Ridley, 2014; Munn, 1986). Experimental approaches manipulating the reliability of alarm callers could be a promising future step.
Coming back to our original question about what birds `talk about´, the information content in alarm calls certainly encompasses only one of many aspects in avian communication. Yet, these studies support the notion that examining the socio-cognitive underpinnings of call-based communication in birds is a promising endeavour (Lambert, Jacobs, Osvath, & Von Bayern, 2019). If we eventually manage to examine the information content (such as individual attributes, motivations, affective states, functional reference to external events) of various calls individuals of a species respond to, we may end up with a relatively complex picture on the receiver side, just as Pepperberg´s pioneering Alex studies defined the realm of be possibility on the production side.
Data Availability
The data and materials for the experiments will be available upon publication.
References
Barton, K. (2019). MuMIn: Multi-Model Inference. Retrieved from https://cran.r-project.org/package=MuMIn
Bates, D., Mächler, M., Bolker, B. M., & Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1). https://doi.org/10.18637/jss.v067.i01
Bílá, K., Beránková, J., Veselý, P., Bugnyar, T., & Schwab, C. (2017). Responses of urban crows to con- and hetero-specific alarm calls in predator and non-predator zoo enclosures. Animal Cognition, 20(1), 43–51. https://doi.org/10.1007/s10071-016-1047-5
Blum, C. R., Fitch, W. T., & Bugnyar, T. (2020). Rapid learning and long-term memory for dangerous humans in ravens (Corvus corax). Frontiers in Psychology, 11, 2843.
Blumstein, D. T., & Daniel, J. C. (2004). Yellow-bellied marmots discriminate between the alarm calls of individuals and are more responsive to calls from juveniles. Animal Behaviour, 68(6), 1257–1265. https://doi.org/10.1016/j.anbehav.2003.12.024
Blumstein, D. T., & Munos, O. (2005). Individual, age and sex-specific information is contained in yellow-bellied marmot alarm calls. Animal Behaviour, 69, 353–361. https://doi.org/10.1016/j.anbehav.2004.10.001
Blumstein, D. T., & Récapet, C. (2009). The sound of arousal: The addition of novel non-linearities increases responsiveness in marmot alarm calls. Ethology, 115(11), 1074–1081. https://doi.org/10.1111/j.1439-0310.2009.01691.x
Boeckle, M., Szipl, G., & Bugnyar, T. (2012). Who wants food? Individual characteristics in raven yells. Animal Behaviour, 84(5), 1123–1130. https://doi.org/10.1016/j.anbehav.2012.08.011
Bolhuis, J. J., & Gahr, M. (2006). Neural mechanisms of birdsong memory. Nature Reviews Neuroscience, 7(5), 347–357. https://doi.org/10.1038/nrn1904
Bolhuis, J. J., Okanoya, K., & Scharff, C. (2010). Twitter evolution: Converging mechanisms in birdsong and human speech. Nature Reviews Neuroscience, 11(11), 747–759. https://doi.org/10.1038/nrn2931
Botham, M. S., Hayward, R. K., Morrell, L. J., Croft, D. P., Ward, J. R., Ramnarine, I., & Krause, J. (2008). Risk-sensitive antipredator behavior in the Trinidadian Guppy, Poecilia reticulata. Ecology, 89(11), 3174–3185. https://doi.org/10.1890/07-0490.1
Boucherie, P. H., Loretto, M., Massen, J. J. M., & Bugnyar, T. (2019). Lessons from ravens What constitutes “social complexity” and “social intelligence” in birds ? Lessons from ravens. Behavioral Ecology and Sociobiology, (January).
Braun, A., & Bugnyar, T. (2012). Social bonds and rank acquisition in raven nonbreeder aggregations. Animal Behaviour, 84(6), 1507–1515. https://doi.org/10.1016/j.anbehav.2012.09.024
Braun, A., Walsdorff, T., Fraser, O. N., & Bugnyar, T. (2012). Socialized sub-groups in a temporary stable raven flock? Journal of Ornithology, 153(1), 97–104. Retrieved from https://doi.org/10.1007/s10336-011-0810-2
Bugnyar, T., & Kotrschal, K. (2002). Scrounging tactics in free-ranging ravens, Corvus corax. Ethology, 108(11), 993–1009. https://doi.org/10.1046/j.1439-0310.2002.00832.x
Catchpole, C. K., & Slater, P. J. B. (2008). Bird Song Biological Themes and Variations. Cambridge University Press. https://doi.org/10.1017/CBO9781107415324.004
Coomes, J. R., Mcivor, G. E., Thornton, A., Coomes, J. R., & Thornton, A. (2019). Evidence for individual discrimination and numerical assessment in collective antipredator behaviour in wild jackdaws ( Corvus monedula ). Biology Letters.
Curio, E. (1978). The Adaptive Significance of Avian Mobbing: I. Teleonomic Hypotheses and Predictions. Zeitschrift Für Tierpsychologie, 48(2). https://doi.org/10.1111/j.1439-0310.1978.tb00254.x
Davidkova, M., Veselý, P., Syrova, M., Nacarovà, J., & Bugnyar, T. (2020). Ravens respond to unfamiliar corvid alarm calls. Journal of Ornithology, Accepted for publication.
Evans, C. S., Evans, L., & Marler, P. (1993). On the meaning of alarm calls: functional reference in an avian vocal system. Animal Behaviour. https://doi.org/10.1006/anbe.1993.1158
Fernández-Juricic, E. (2012). Sensory basis of vigilance behavior in birds: Synthesis and future prospects. Behavioural Processes, 89(2), 143–152. https://doi.org/10.1016/j.beproc.2011.10.006
Fichtel, C., & Van Schaik, C. P. (2006). Semantic differences in sifaka (Propithecus verreauxi) alarm calls: A reflection of genetic or cultural variants? Ethology, 112(9), 839–849. https://doi.org/10.1111/j.1439-0310.2006.01239.x
Flower, T. P., Gribble, M., & Ridley, A. R. (2014). Deception by Flexible Alarm Mimicry in an African Bird. Science, 344(6183), 513 LP–516. https://doi.org/10.1126/science.1249723
Foster, W. A., & Treherne, J. E. (1981). Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature, 293(5832), 466–467. https://doi.org/10.1038/293466a0
Gallego-Abenza, M., Loretto, M.-C., & Bugnyar, T. (2020). Decision time modulates social foraging success in wild common ravens , Corvus corax. Ethology, (126), 413–422. https://doi.org/10.1111/eth.12986
Gill, S. A., & Bierema, A. M. K. (2013). On the Meaning of Alarm Calls: A Review of Functional Reference in Avian Alarm Calling. Ethology, 119(6), 449–461. https://doi.org/10.1111/eth.12097
Graw, B., & Manser, M. B. (2007). The function of mobbing in cooperative meerkats. Animal Behaviour, 74(3). https://doi.org/10.1016/j.anbehav.2006.11.021
Griesser, M. (2008). Referential Calls Signal Predator Behavior in a Group-Living Bird Species. Current Biology, 18(1), 69–73. https://doi.org/10.1016/j.cub.2007.11.069
Griesser, M. (2013). Do warning calls boost survival of signal recipients? Evidence from a field experiment in a group-living bird species. Frontiers in Zoology, 10(1), 1. https://doi.org/10.1186/1742-9994-10-49
Griesser, M., & Ekman, J. (2004). Nepotistic alarm calling in the Siberian jay, Perisoreus infaustus. Animal Behaviour, 67(5), 933–939. https://doi.org/10.1016/j.anbehav.2003.09.005
Griesser, M., & Ekman, J. (2005). Nepotistic mobbing behaviour in the Siberian jay, Perisoreus infaustus. Animal Behaviour, 69(2), 345–352. https://doi.org/10.1016/j.anbehav.2004.05.013
Guillemain, M., Duncan, P., & Fritz, H. (2001). Switching to a feeding method that obstructs vision increases head-up vigilance in dabbling ducks. Journal of Avian Biology, 32(4), 345–350. https://doi.org/10.1111/j.0908-8857.2001.320409.x
Hare, J. F. (1998). Juvenile Richardson’s ground squirrels, Spermophilus richardsonii, discriminate among individual alarm callers. Animal Behaviour, 55(2), 451—460. https://doi.org/10.1006/anbe.1997.0613
Hare, J. F., & Atkins, B. A. (2001). The squirrel that cried wolf: Reliability detection by juvenile Richardson’s ground squirrels (Spermophilus richardsonii). Behavioral Ecology and Sociobiology, 51(1), 108–112. https://doi.org/10.1007/s002650100414
Hauser, M. D. (1996). The evolution of communication. The MIT Press. Cambridge, MA, US: The MIT Press.
Heinrich, B., & Marzluff, J. (1995). Why ravens share. American Scientist, 83(4), 342–349. Retrieved December 16, 2020, from http://www.jstor.org/stable/29775481
Heinrich, B., & Pepper, J. W. (1998). Influence of competitors on caching behaviour in the common raven, Corvus corax. Animal Behaviour, 56(5), 1083–1090. https://doi.org/10.1006/anbe.1998.0906
Heinrich, Bernd. (1989). Ravens in Winter. (Summit Books of Simon & Schuster, Ed.) (New York). Vintage Books. Retrieved from https://books.google.at/books?id=YbWtadkEVvUC
Kalb, N., Anger, F., & Randler, C. (2019). Subtle variations in mobbing calls are predator-specific in great tits (Parus major). Scientific Reports, 9(1), 1–7. https://doi.org/10.1038/s41598-019-43087-9
Kotler, B. P., Blaustein, L., & Brown, J. S. (1992). Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Annales Zoologici Fennici.
Krams, I., Krama, T., Igaune, K., & Mänd, R. (2008). Experimental evidence of reciprocal altruism in the pied flycatcher. Behavioral Ecology and Sociobiology, 62(4), 599–605. https://doi.org/10.1007/s00265-007-0484-1
Lambert, M. L., Jacobs, I., Osvath, M., & Von Bayern, A. M. P. (2019). Birds of a feather? Parrot and corvid cognition compared. Behaviour, 156(5–8), 505–594. https://doi.org/10.1163/1568539X-00003527
Leavesley, A. J., & Magrath, R. D. (2005). Communicating about danger: Urgency alarm calling in a bird. Animal Behaviour, 70(2), 365–373. https://doi.org/10.1016/j.anbehav.2004.10.017
Lohrey, A. K., Clark, D. L., Gordon, S. D., & Uetz, G. W. (2009). Antipredator responses of wolf spiders (Araneae: Lycosidae) to sensory cues representing an avian predator. Animal Behaviour, 77(4), 813–821. https://doi.org/10.1016/j.anbehav.2008.12.025
Loretto, M.-C., Schuster, R., & Bugnyar, T. (2016). GPS tracking of non-breeding ravens reveals the importance of anthropogenic food sources during their dispersal in the Eastern Alps. Current Zoology, 62(4), 337–344. https://doi.org/10.1093/cz/zow016
Loretto, M.-C., Schuster, R., Itty, C., Marchand, P., Genero, F., & Bugnyar, T. (2017). Fission-fusion dynamics over large distances in raven non-breeders. Scientific Reports, 7(1), 380. Retrieved from https://doi.org/10.1038/s41598-017-00404-4
Magrath, R. D., Pitcher, B. J., & Gardner, J. L. (2009). Recognition of other species’ aerial alarm calls: speaking the same language or learning another? Proceedings. Biological Sciences / The Royal Society, 276(February), 769–774. https://doi.org/10.1098/rspb.2008.1368
Marler, P. (2004). Bird Calls: Their Potential for Behavioral Neurobiology. Annals of the New York Academy of Sciences, 1016(1), 31–44. https://doi.org/10.1196/annals.1298.034
Munn, C. A. (1986). Birds that ‘cry wolf.’ Nature, 319(6049), 143–145. https://doi.org/10.1038/319143a0
Nácarová, J., Veselý, P., & Bugnyar, T. (2018). Ravens adjust their antipredatory responses to con- and specific alarms to the perceived threat. Ethology, (May), 1–8. https://doi.org/10.1111/eth.12764
Parker, P. G., Waite, T. A., Heinrich, B., & Marzluff, J. M. (1994). Do common ravens share ephemeral food resources with kin? DNA fingerprinting evidence. Animal Behaviour, 48(5), 1085–1093. https://doi.org/10.1006/anbe.1994.1342
Pepperberg, I. M. (1983). Cognition in the African Grey parrot: Preliminary evidence for auditory/vocal comprehension of the class concept. Animal Learning & Behavior, 11(2), 179–185. https://doi.org/10.3758/BF03199646
Pepperberg, I. M. (1990). Cognition in an African gray parrot (Psittacus erithacus): Further evidence for comprehension of categories and labels. Journal of Comparative Psychology, 104(1), 41–52. https://doi.org/10.1037/0735-7036.104.1.41
Pepperberg, I. M. (1999). The Alex Studies. Harvard University Press. https://doi.org/10.2307/j.ctvk12qc1
Pepperberg, I. M. (2002). Cognitive and communicative abilities of grey parrots. Current Directions in Psychological Science, 11(3), 83–87. https://doi.org/10.1111/1467-8721.00174
Pepperberg, I. M. (2008). Peer-reviewed parrot studies speak for themselves, as he did. Nature, 456(7219), 166. https://doi.org/10.1038/456166a
R Development Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Ramakrishnan, U., & Coss, R. G. (2000). Age differences in the responses to adult and juvenile alarm calls by bonnet macaques (Macaca radiata). Ethology, 106(2), 131–144. https://doi.org/10.1046/j.1439-0310.2000.00501.x
Rendall, D., Owren, M. J., & Ryan, M. J. (2009). What do animal signals mean? Animal Behaviour, 78(2), 233–240. https://doi.org/10.1016/j.anbehav.2009.06.007
Scarpignato, A. L., & George, T. L. (2011). Home range and habitat use of breeding common ravens in Redwood National and State Parks.
Searle, S. R., Speed, F. M., & Milliken, G. A. (1980). Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. The American Statistician, 34(4), 216–221. https://doi.org/10.1080/00031305.1980.10483031
Seyfarth, R. M., & Cheney, D. L. (1986). Vocal development in vervet monkeys. Animal Behaviour, 34(6), 1640–1658. https://doi.org/10.1016/S0003-3472(86)80252-4
Seyfarth, R. M., Cheney, D. L., & Marler, P. (1980). Vervet monkey alarm calls: Semantic communication in a free-ranging primate. Animal Behaviour, 28(4), 1070–1094. https://doi.org/10.1016/S0003-3472(80)80097-2
Shedd, D. (1982). Seasonal Variation and Function of Mobbing and Related Antipredator Behaviors of the American Robin (Turdus migratorius). The Auk: Ornithological Advances, 99(2), 342–346. https://doi.org/10.1093/auk/99.2.342
Shields, W. M. (1984). Barn swallow mobbing: Self-defence, collateral kin defence, group defence, or parental care? Animal Behaviour, 32(1), 132–148. https://doi.org/10.1016/S0003-3472(84)80331-0
Sloan, J. L., & Hare, J. F. (2008). The More the Scarier: Adult Richardson’s Ground Squirrels (Spermophilus richardsonii) Assess Response Urgency Via the Number of Alarm Signallers. Ethology, 114(5), 436–443. https://doi.org/10.1111/j.1439-0310.2008.01479.x
Smith, J. M. (1965). The evolution of alarm calls. The American Naturalist, 99(904), 59–63. Retrieved from http://www.jstor.org/stable/2459256
Suzuki, T. N. (2011). Parental alarm calls warn nestlings about different predatory threats. Current Biology, 21(1), R15–R16. https://doi.org/10.1016/j.cub.2010.11.027
Suzuki, T. N. (2012). Referential mobbing calls elicit different predator-searching behaviours in Japanese great tits. Animal Behaviour, 84(1), 53–57. https://doi.org/10.1016/j.anbehav.2012.03.030
Suzuki, T. N. (2014). Communication about predator type by a bird using discrete, graded and combinatorial variation in alarm calls. Animal Behaviour, 87, 59–65. https://doi.org/10.1016/j.anbehav.2013.10.009
Suzuki, T. N., Wheatcroft, D., & Griesser, M. (2016). Experimental evidence for compositional syntax in bird calls. Nature Communications, 7, 1–7. https://doi.org/10.1038/ncomms10986
Swan, D. C., & Hare, J. F. (2008). Signaler and Receiver Ages Do Not Affect Responses to Richardson’s Ground Squirrel Alarm Calls. Journal of Mammalogy, 89(4), 889–894. https://doi.org/10.1644/07-mamm-a-228.1
Szipl, G., Ringler, E., & Bugnyar, T. (2018). Attacked ravens flexibly adjust signalling behaviour according to audience composition. Proceedings of the Royal Society B, 285(June). https://doi.org/10.1098/rspb.2018.0375
Szipl, G., Ringler, E., Spreafico, M., & Bugnyar, T. (2017). Calls during agonistic interactions vary with arousal and raise audience attention in ravens. Frontiers in Zoology, 14(1). https://doi.org/10.1186/s12983-017-0244-7
Tibbetts, E. A., & Dale, J. (2007). Individual recognition: it is good to be different. Trends in Ecology and Evolution, 22(10), 529–537. https://doi.org/10.1016/j.tree.2007.09.001
Tornick, J. K., Callahan, E. S., & Gibson, B. M. (2015). An investigation of quantity discrimination in Clark’s nutcrackers (Nucifraga columbiana). Journal of Comparative Psychology. Tornick, Jan K.: Department of Psychology, University of New Hampshire, 10 Library Way, Durham, NH, US, 03824, j.tornick@unh.edu: American Psychological Association. https://doi.org/10.1037/a0037863
Townsend, S. W., Rasmussen, M., Clutton-Brock, T., & Manser, M. B. (2012). Flexible alarm calling in meerkats: The role of the social environment and predation urgency. Behavioral Ecology, 23(6), 1360–1364. https://doi.org/10.1093/beheco/ars129
Uchida, K., Suzuki, K., Shimamoto, T., Yanagawa, H., & Koizumi, I. (2016). Seasonal variation of flight initiation distance in Eurasian red squirrels in urban versus rural habitat. Journal of Zoology, 298(3), 225–231. https://doi.org/10.1111/jzo.12306
Webb, W. C., Boarman, W. I., & Rotenberry, J. T. (2004). Common Raven Juvenile Survival in a Human-Augmented Landscape. The Condor, 106(3), 517–528. https://doi.org/10.1093/condor/106.3.517
Wheatcroft, D., Gallego-Abenza, M., & Qvarnström, A. (2016). Species replacement reduces community participation in avian antipredator groups. Behavioral Ecology, 00, arw074. https://doi.org/10.1093/beheco/arw074
Wheatcroft, D., & Price, T. D. (2008). Reciprocal cooperation in avian mobbing: playing nice pays. Trends in Ecology and Evolution, 23(8), 416–419. https://doi.org/10.1016/j.tree.2008.04.011
Woods, R. D., Kings, M., McIvor, G. E., & Thornton, A. (2018). Caller characteristics influence recruitment to collective anti-predator events in jackdaws. Scientific Reports, 8(1), 1–8. https://doi.org/10.1038/s41598-018-25793-y
Zuberbühler, K., Noë, R., & Seyfarth, R. M. (1997). Diana monkey long-distance calls: Messages for conspecifics and predators. Animal Behaviour, 53(3), 589–604. https://doi.org/10.1006/anbe.1996.0334
Acknowledgements
The present study was funded by the Austrian Science Fund (FWF) project P29705 and an IP project IP550002 of the Faculty of Life Sciences, University of Vienna; permanent support is granted by the ‘Verein der Förderer KLF’ and the Faculty of Life Sciences, University of Vienna. CB was supported by the uni:docs stipend of University of Vienna. We thank our colleagues Dr. Petra Sumasgutner and Dr. Mauricio Nicolas Adreani for their help in the statistical analyses and Noelia Abenza for her kind help editing figures. We also thank the Cumberland Wildpark Grünau for the excellent cooperation while conducting this study.
Funding
Open Access funding provided by University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallego-Abenza, M., Blum, C.R. & Bugnyar, T. Who is crying wolf? Seasonal effect on antipredator response to age-specific alarm calls in common ravens, Corvus corax. Learn Behav 49, 159–167 (2021). https://doi.org/10.3758/s13420-020-00455-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-020-00455-0