Abstract
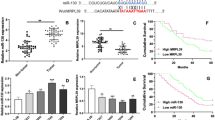
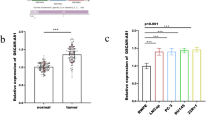
Increasing numbers of evidences have demonstrated that microRNAs (miRNAs) play an important role in osteosarcoma (OS) cell functions. MiR-501-3p has been reported to play an important role in several types of tumors, including prostate cancer and hepatocellular carcinoma. However, the biological function and potential mechanism of miR-501-3p in OS have not been well investigated until now. Here, we analyzed the expression of miR-501-3p in OS tissues and cell lines and its clinical significance in OS patients. Quantitative reverse transcription PCR showed miR-501-3p was significantly up-regulated in OS tissues and cell lines. Up-regulated miR-501-3p expression was associated with TNM stage, distal metastasis and worse prognosis in OS patients. MiR-501-3p knockdown and overexpression were achieved by miR-501-3p inhibitor and mimics transfection, respectively. CCK-8, colony formation and transwell assays showed that miR-501-3p knockdown in U2OS and Saos-2 cells suppressed, while miR-501-3p overexpression in Saos-2 cells promoted cell proliferation, migration and invasion. Moreover, luciferase reporter assay supporting BCL7A was a target of miR-501-3p and its expression was increased by miR-501-3p inhibitor, but inhibited by miR-501-3p mimics. By performing rescue experiments, we further demonstrated that BCL7A was a downstream functional regulator involved in miR-501-3p promoting OS cell functions. In summary, our findings suggest that miR-501-3p targets BCL7A may provide novel therapeutic targets for the treatment of OS.






Similar content being viewed by others
Abbreviations
- OS:
-
Osteosarcoma
- 3′-UTR:
-
3′-Untranslated region
- cDNA:
-
Complementary DNA
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- FBS:
-
Fetal bovine serum
- NC:
-
Negative control
- CCK-8:
-
Cell Counting Kit-8
- PVDF:
-
Polyvinylidene fluoride
- TBST:
-
Tris-buffered saline with Tween-20
References
Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34.
Poletajew S, Fus L, Wasiutynski A. Current concepts on pathogenesis and biology of metastatic osteosarcoma tumors. Ortop Traumatol Rehab. 2011;13:537–45.
Gorlick R. Current concepts on the molecular biology of osteosarcoma. Cancer Treat Res. 2009;152:467–78.
He X, Gao Z, Xu H, Zhang Z, Fu P. A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J Orthop Surg Res. 2017;12:5.
Anninga JK, Gelderblom H, Fiocco M, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer. 2011;47:2431–45.
Taran SJ, Taran R, Malipatil NB. Pediatric osteosarcoma: an updated review. Indian J Med Paediatr Oncol. 2017;38:33–43.
Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38.
Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–64.
Zhang Z, Shao L, Wang Y, Luo X. MicroRNA-501-3p restricts prostate cancer growth through regulating cell cycle-related and expression-elevated protein in tumor/cyclin D1 signaling. Biochem Biophys Res Commun. 2019;509:746–52.
Luo C, Yin D, Zhan H, et al. microRNA-501-3p suppresses metastasis and progression of hepatocellular carcinoma through targeting LIN7A. Cell Death Dis. 2018;9:535.
Lu J, Zhou L, Wu B, et al. MiR-501-3p functions as a tumor suppressor in non-small cell lung cancer by downregulating RAP1A. Exp Cell Res. 2020;387:111752.
Yin Z, Ma T, Huang B, et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38:310.
Huang C, Wang Q, Ma S, Sun Y, Vadamootoo AS, Jin C. A four serum-miRNA panel serves as a potential diagnostic biomarker of osteosarcoma. Int J Clin Oncol. 2019;24:976–82.
Jadayel DM, Osborne LR, Coignet LJ, et al. The BCL7 gene family: deletion of BCL7B in Williams syndrome. Gene. 1998;224:35–44.
Zani VJ, Asou N, Jadayel D, et al. Molecular cloning of complex chromosomal translocation t(8;14;12)(q24.1;q32.3;q24.1) in a Burkitt lymphoma cell line defines a new gene (BCL7A) with homology to caldesmon. Blood. 1996;87:3124–34.
Carbone A, Bernardini L, Valenzano F, et al. Array-based comparative genomic hybridization in early-stage mycosis fungoides: recurrent deletion of tumor suppressor genes BCL7A, SMAC/DIABLO, and RHOF. Genes Chromosom Cancer. 2008;47:1067–75.
van Doorn R, Zoutman WH, Dijkman R, et al. Epigenetic profiling of cutaneous T-cell lymphoma: promoter hypermethylation of multiple tumor suppressor genes including BCL7a, PTPRG, and p73. J Clin Oncol. 2005;23:3886–96.
Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11.
Martinez-Delgado B, Melendez B, Cuadros M, et al. Expression profiling of T-cell lymphomas differentiates peripheral and lymphoblastic lymphomas and defines survival related genes. Clin Cancer Res. 2004;10:4971–82.
Potter N, Karakoula A, Phipps KP, et al. Genomic deletions correlate with underexpression of novel candidate genes at six loci in pediatric pilocytic astrocytoma. Neoplasia. 2008;10:757–72.
Yu N, Shin S, Choi JR, Kim Y, Lee KA. Concomitant AID expression and bcl7a loss associates with accelerated phase progression and imatinib resistance in chronic myeloid leukemia. Ann Lab Med. 2017;37:177–9.
Sun Z, Sun L, He M, Pang Y, Yang Z, Wang J. Low BCL7A expression predicts poor prognosis in ovarian cancer. J Ovarian Res. 2019;12:41.
Jiang X, Wang W, Yang Y, et al. Identification of circulating microRNA signatures as potential noninvasive biomarkers for prediction and prognosis of lymph node metastasis in gastric cancer. Oncotarget. 2017;8:65132–42.
Giunti L, Da Ros M, De Gregorio V, et al. A microRNA profile of pediatric glioblastoma: The role of NUCKS1 upregulation. Mol Clin Oncol. 2019;10:331–8.
Larsen AC. Conjunctival malignant melanoma in Denmark: epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol. 2016;94(1):1–27.
Litvinov IV, Zhou Y, Kupper TS, Sasseville D. Loss of BCL7A expression correlates with poor disease prognosis in patients with early-stage cutaneous T-cell lymphoma. Leuk Lymphoma. 2013;54:653–4.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, J., Lu, L., Kang, L. et al. MiR-501-3p promotes osteosarcoma cell proliferation, migration and invasion by targeting BCL7A. Human Cell 34, 624–633 (2021). https://doi.org/10.1007/s13577-020-00468-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00468-x