Abstract
In this study, the interannual variations of ichthyoplankton assemblages in the Taiwan Strait (TS) during the winters of 2007–2013 were determined. The cold China Coastal Current (CCC) and Mixed China Coastal Water (MCCW) intruded into the TS and impinged with the warm Kuroshio Branch Current (KBC) with annual variations. Consequently, the ichthyoplankton community in the TS was mainly structured into two assemblages characterized by differing environmental conditions. The composition of the warm KBC assemblage was relatively stable and was characterized by Diaphus B and Bregmaceros spp. By contrast, the cold MCCW assemblage demonstrated considerable variations over the years, with demersal Gobiidae and Scorpaenidae families considered the most representative. In addition, Benthosema pterotum and Trichiurus spp. were common in both KBC and MCCW assemblages. The distribution of the KBC assemblage demonstrated sharp boundaries in the frontal zones, whereas changes in the assemblage structure between the frontal zones were gradual for the MCCW assemblage, particularly when demersal taxa were dominant. Sea surface temperature and salinity were most strongly associated with variability in the assemblage structure during the study period. Thus, this paper provides a better understanding of long-term larval fish dynamics during winter in the TS.
Similar content being viewed by others
Introduction
The early life stage is crucial for determining annual recruitment in fish populations (Shepherd and Cushing 1980). Ichthyoplankton comprise fish eggs and larvae; they represent a planktonic stage that is highly sensitive to environmental changes. Most processes determining the recruitment strength and spatial distribution of fish populations occur during the planktonic stage, and the survival rate of larval fish may result in significant interannual fluctuations in adult fish stocks (Busby et al. 2014; Guan et al. 2017). Many studies have focused on changes in the composition and spatiotemporal distribution of larval fish assemblages in relation to dynamic physical processes, including oceanographic features (Okazaki and Nakata 2007; Atwood et al. 2010; Norcross et al. 2010), as well as biological factors such as the abundance of phytoplankton and zooplankton (Sanvicente-Añorve et al. 2006; Hsieh et al. 2012). In particular, information on the distribution and abundance of fish eggs and larvae can provide evidence of spawning grounds and environmental requirements for important fish species. Furthermore, the linkage between larval fish assemblages and physical–biological processes is becoming increasingly essential to ecosystem-based fishery management and fishery-independent stock assessments (Hsieh et al. 2012; Busby et al. 2014; Guan et al. 2017).
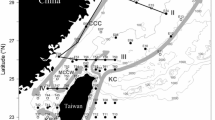
The hydrography of the waters surrounding Taiwan is complex (Fig. 1). The Kuroshio Current (KC) flows continuously northward along the eastern coast of Taiwan. During the northeasterly monsoon in winter, with its intrusion along the edge of the continental shelf, the KC becomes the Kuroshio Branch Current of Northern Taiwan (KBCNT), which transports warm and saline Kuroshio waters into the East China Sea (ECS) (Gong et al. 1996). To the west, the Taiwan Strait (TS) is a shallow strait approximately 180 km wide, 350 km long, and 60 m in average depth. A distinct special topographic feature in the TS is the Chang-Yuen Ridge (CYR), extending westward from the middle of the west coast of Taiwan. During winter, the cold and low-salinity China Coastal Current (CCC) is driven southward and intrudes into the TS (Chen and Wang 2006). Moreover, a branch of the CCC turns northward along the northern coast of Taiwan because of the topographic effect of the CYR, which then becomes the Mixed China Coastal Water (MCCW). Therefore, the Kuroshio Front (KF) is formed between the MCCW and warm KBCNT (Chang et al. 2006). In the southern TS, the KC intrudes into the South China Sea (SCS) through the Luzon Strait, which then becomes the Kuroshio Branch Current (KBC) and flows northward into the TS (Hsieh et al. 2012). The warm KBC impinges the cold MCCW in the middle of the TS, and sharp frontal bands are formed along the Peng-Hu Channel extending northward around the CYR. This frontal area in the middle of the TS is referred to as the Peng-Chang Front (PCF). Therefore, the TS is characterized by strong hydrodynamic front effects caused by the interaction between these currents.
Locations of the sampling stations in the TS during the winters of 2007–2013. The gray dotted line illustrates the transect for the vertical profiles of temperature and salinity. Gray arrows illustrate the summary view of current circulation, and dark gray lines indicate the frontal area in wintertime, redrawn from Jan et al. (2006), Chang et al. (2006), and Chang et al. (2008). CYR Chang-Yuen Ridge, CCC China Coastal Current, MCCW Mixed China Coastal Water, KC Kuroshio Current, KBC Kuroshio Branch Current, KBCNT Kuroshio Branch Current of northern Taiwan, KF Kuroshio Front, PCF Peng-Chang Front
Several studies have focused on the relationship between larval fish assemblages and environmental factors in waters off Taiwan (Online Resource, Table S1). Hsieh et al. (2007) studied the composition and spatial distribution of larval fish in the waters surrounding Taiwan during winter. Other studies have examined the effects of monsoon systems on ichthyoplankton and the circulation patterns in the TS (Hsieh et al. 2011, 2012). These studies have suggested that the larval fish assemblage under the influence of the warm KBC was dominated by Sigmops gracilis and Vinciguerria nimbaria in the TS, whereas that under the influence of cold CCC was dominated by Engraulis japonicus, Scomber spp., Callionymidae, and Carangoides ferdau. Wang et al. (2013) showed that in the northern TS during winter, the ichthyoplankton assemblage influenced by TS waters was dominated by Saurida spp., Trichiurus lepturus, and Bregmaceros spp., and that Sebastiscus marmoratus was predominant in the assemblage influenced by the CCC. Furthermore, larval fish assemblages during winter in both the ECS and waters surrounding Taiwan have been described (Chen et al. 2016). S. gracilis and Diaphus B dominated the KBC assemblage, and Trichiurus spp. and Scorpaenidae dominated the MCCW assemblage. However, most previous descriptions on larval fish in the TS have been derived from a single survey; none of the aforementioned studies have maintained a long-duration series to provide sufficient information on the interannual variability of larval fish assemblages in the TS.
Understanding the environmental effects on larval fish populations is essential for the ecosystem-based management of fisheries. However, assessing fishery population dynamics through sampling for 1 or several years might provide false results. Moreover, information on larval fish assemblages in the waters surrounding Taiwan during winter remains minimal because during the northeasterly monsoon, rough sea conditions impede fieldwork, particularly long-term observations. In the present study, we specifically examined interannual variations in the larval fish assemblage in winters of 2007–2013 to explore the response of larval fish to the environmental changes in the TS. Our principal objectives were to (1) describe the larval fish assemblages in the TS during winter, (2) identify key species most representative of the assemblage and water masses, (3) examine the interannual variations in spatial extent of the ichthyoplankton assemblages, and (4) investigate the relationships between the larval fish assemblage patterns and physical oceanographic variables. Our overall aim was to provide baseline information on the structure of larval fish assemblages in the TS, allowing for a better understanding of long-term larval fish dynamics and their relation to environmental fluctuations, which may be used to evaluate the influence of climate change on future ichthyoplankton composition in the TS.
Materials and methods
Field sampling
Ichthyoplankton samples were collected from 28 stations in the TS (Fig. 1), surveyed in January during seven consecutive years from 2007–2013 aboard Fishery Researcher I. A transect was employed to illustrate the vertical profiles of temperature and salinity along the stations. For ichthyoplankton samples, an Ocean Research Institute net with a mouth diameter of 160 cm and mesh size of 330 μm was towed at the speed of 1 m/s obliquely from a depth of 200 m to the surface at deep stations or from 5 m above the sea floor to the surface at shallow stations. A General Oceanics flowmeter was suspended across the center of the net mouth to measure the amount of water filtered during each tow. Samples were immediately preserved in 5% buffered formalin seawater. In the laboratory, using a Folsom splitter, each sample was divided into two subsamples. Ichthyoplankton species were sorted from one subsample and identified to the lowest taxonomic level whenever possible. To calculate the abundance of zooplankton, the other subsample was repeatedly divided until 1000–2000 individual zooplankton remained. Temperature and salinity were recorded using a CTD profiler (Seabird SBE-911 Plus) lowered from the surface to a depth of 200 m (if possible) at each station. A Rosette (GO-1015) multi-bottle array mounted on the frame of the CTD profiler was sequentially closed at specific target depths as the CTD profiler was raised. Seawater (1 L) was filtered through Whatman GF/F filter papers and stored in a freezer. In the laboratory, the integrated chlorophyll a concentration of the euphotic zone was determined using trichromatic equations (Jeffrey and Humphrey 1975).
Data analysis
The abundance of larval fish and zooplankton was standardized as the number of larvae (ind.) per 1000 m3 and 1 m3, respectively. Hierarchical cluster analysis in conjunction with nonmetric multidimensional scaling (MDS) ordinations was used to identify potential larval fish assemblages. Only species accounting for ≥ 1% of the total number of specimens were included in the analysis. Samples lacking larval fish were not included in the cluster analysis. Larval fish abundance was log(X + 1)-transformed to normalize the data and homogenize residual variances, and the Bray–Curtis similarity matrix was generated (Bray and Curtis 1957). Moreover, group-average linking was used to delineate groups with distinct community structures. Nonmetric MDS ordinations were also used to provide a two-dimensional visual representation of the assemblage structure. Similarity percentage analysis (SIMPER) was employed to identify the taxa contributing the most to the differences and typifying each assemblage (Clark 1993). A nonparametric multivariate BIO-ENV procedure was used to analyze the relationship between environmental variables and larval fish assemblage structures (Clarke and Warwick 2001; Clarke and Gorley 2006). This process selects abiotic variables that maximize the Spearman correlation (ρw) between the biotic and abiotic similarity matrices (Bray–Curtis for biota, and Euclidean distance for environmental variables). Multivariate analyses were performed using PRIMER (version 6.0).
Results
Hydrographic conditions
In general, lower sea surface temperature (SST) was observed in the northern TS (Fig. 2a). The coldest water under the influence of the CCC was observed along the Chinese coast; SST increased from the Chinese coast outward toward Taiwan in the northern TS (Fig. 2a). In 2008, 2009, and 2010, the influence of cold water masses was weak, and the warm KBC was the strongest among the cruises, flowing northward across the Penghu Islands to the northern TS; the coldest CCC (< 16.5 °C) was almost absent in the northern TS. The cold MCCW (< 19.8 °C) was also confined to the north of the CYR. In 2011, 2012, and 2013, cold water masses were the strongest, where CCC evidently occupied the middle TS, and MCCW intruded southward over the Penghu Islands to the southern TS, impinged with the warm KBC. The sea surface salinity (SSS) gradient was also evident (Fig. 2a). In 2008, 2009, and 2010, the CCC was weak, and the MCCW demonstrated that low-salinity water (< 33.9 psu) retreated to the north of the CYR. In 2007, 2011, and 2013, the influence of low-salinity water reached the Penghu Islands in the middle of the TS. In 2012, low-salinity waters intruded southmost to the southern TS, where the high-salinity KBC was confined.
The vertical profiles showed no stratifications in the TS during winter (Fig. 3) because the water columns were well mixed due to strong monsoons. In 2007, the cold MCCW reached the middle of the TS, with a strong gradient observed between stations 44 and 42; moreover, the MCCW impinged with the KBCNT between stations 50 and 54 in the northern TS. In 2008 and 2009, the MCCW retreated to the northern TS, with a strong gradient observed between stations 47 and 44; moreover, the MCCW impinged with the KBCNT between stations 54 and 56 in the northern TS. Similar patterns were noted in 2010, with differences in the cold MCCW reaching station 56 in the north. In 2011, the CCC occupied areas around station 47. In 2012, the CCC reached station 44; the cold water masses were the strongest and occupied stations 42, 37, and 38 in the southern TS. In 2013, cold water occupied stations 44–54, whereas the CCC occurred at station 44.
The mean chlorophyll a concentrations of each cruise ranged from 0.176 to 0.339 mg/m3 in 2012 and 2008, respectively. In general, the distribution of chlorophyll a revealed higher concentrations in the waters south of the Penghu Islands during the cruises (Fig. 2b). Increased concentrations occasionally occurred in the northern TS. The mean zooplankton abundance varied from 87 to 293 ind./m3 in 2012 and 2007, respectively. The overall mean was 173 ind./m3. In general, the distribution of zooplankton abundance was relatively higher in the warm KBC for each cruise (Fig. 2b), except in 2008 and 2009. For larval fish, the mean abundance varied from 40 to 118 ind./1000 m3 in 2011 and 2013, respectively. In general, larval fish showed higher abundance in the waters south of the Penghu Islands, where the warm KBC occurred, during the cruises (Fig. 2c). By contrast, in the north of Penghu Islands, where the MCCW occurred, ichthyoplankton was absent in many stations in 2007 and 2009–2012.
In the present study, we plotted the T–S diagram to determine water masses for each station (Online Resource, Fig. S1) according to the temperature and salinity defined by studies concerning the typical ranges of the water masses of the study area (Gong et al. 1996; Ichikawa and Chaen 2000; Jan et al. 2006). Based on the previous studies, the stations were classified into three categories: CCC (< 16.5 °C; 30.7–33.7 psu), MCCW (16.5–19.8 °C; 33.0–33.9 psu), and KC (all uncategorized stations with higher temperature and salinity, including KBC and KBCNT). The boundaries of different water masses were drawn according to the water masses defined for each station to demonstrate the frontal areas for each cruise (Fig. 5). The results showed that the frontal zone was evident in the middle of the TS with annual variability.
Ichthyoplankton assemblages
In total, 5774 specimens of larval fish were identified, belonging to 143 taxa in 80 families. Among the 80 families, 23 taxa exceeded 1% of the total larval fish catch, accounting for 71.5% of the total fish larvae. Cluster analysis, based on the larval fish compositions of each cruise, defined the four main groups of stations during the 7-year surveys at similarity levels of 9–20% (Fig. 4). The MDS diagram derived from larval fish species composition indicates clear structures, in which data from stations clustered together. In 2007, sampling stations were divided into four groups (Fig. 4), and each group was named according to their major areas of occurrence of water masses. The first group comprised two sampling stations (stations 43 and 44), as did the second group (stations 41 and 46); both were under the influence of the MCCW (Online Resource, Fig. S1) and were therefore named MCCW assemblage (M) and MCCW2 assemblage (M2), respectively. The third group comprised nine stations under the influence of the KBC and was named the KBC assemblage (K). The fourth group (stations 55 and 56) under the influence of the KBCNT was further defined as the KBCNT assemblage (K2). In 2008, two main groups of stations were distinguished (Fig. 4), that is, K and M assemblages. Similarly, the larval fish assemblages in 2009, 2010, 2011, 2012, and 2013 cruises were classified into K and M; K, M, and M2; K and M; K and M; and K, M, and M2, respectively.
Hierarchical clustering and multidimensional scaling (MDS) ordination of the station assemblages based on Bray–Curtis similarity matrix of log(X + 1)-transformed abundance of larval fish in the TS during the winters of 2007–2013. For visual clarity, single (ungrouped) stations in the dendrogram of 2008, 2011, and 2012 are not shown on the MDS plot. K Kuroshio Branch Current assemblage, K2 Kuroshio Branch Current of Northern Taiwan assemblage, M Mixed China Coastal Water assemblage, M2 Mixed China Coastal Water assemblage 2
The top five abundant taxa of each assemblage derived from cluster analysis for each cruise are shown in Table 1. For the K assemblage, the composition was relatively stable throughout the years, and mesopelagic fishes were the most common taxa among the cruises. The occurrence rate of Diaphus B in the K assemblage was 100% (i.e., present as the dominant taxa in each of the seven cruises). The second most frequently occurring species was Benthosema pterotum (83.3%). Bregmaceros spp. and Trichiurus spp. were also common among the cruises, with 57.1% occurrence rates for both. Moreover, 11 taxa were present exclusively in K assemblages, and nine taxa were found only once as the dominant taxa, except for Stomias nebulosus and Lampanyctus spp. The K2 assemblage was found only once in 2007, and the composition of dominant taxa was distinct from that in the K1 assemblage.
For the M and M2 assemblages distinguished from cluster analysis, M2 comprised only 2–3 stations in 2007, 2010, and 2013 (Fig. 4). Moreover, M and M2 had similar environments, that is, under the influence of the cold MCCW. Therefore, we regarded them as a whole for further discussion. For the M and M2 assemblages, the composition of dominant taxa demonstrated drastic variations among the years. The most frequently occurring dominant taxa were B. pterotum and members of the demersal Gobiidae and Scorpaenidae families, all of which accounted for a 57.1% occurrence rate for each taxon. Bregmaceros spp. and Trichiurus spp. were also common among the cruises, with 42.8% occurrence rates for both. Fifteen taxa were present exclusively in M and M2 assemblages; 11 taxa showed only once as dominant taxa.
In addition, B. pterotum was the most common dominant taxon among K, K2, M, and M2 assemblages, and Trichiurus spp. was also common across the survey area (Table 1). Moreover, the most abundant Diaphus B in K assemblages occurred only once as the fourth abundant taxa in the M assemblage in 2010 (single occurrence in station 41). Therefore, we regarded Diaphus B as a good indicator of the intrusion of the KBC into the TS in winter. On the other hand, the most frequently occurring Gobiidae and Scorpaenidae were present exclusively in M and M2 assemblages during the 7 study years. Accordingly, these two demersal taxa were considered good indicators of the MCCW in the TS.
SIMPER analysis
The taxa with the greatest contribution to each larval assemblage in each year are shown in Table 2. The average between-group dissimilarity was generally high, ranging from 88.45 to 100%, for each cruise. Regarding the warm K assemblages in 2007–2013, Diaphus B contributed the most to the within-group similarities in 2008, 2009, 2010, and 2013 and contributed to 12.5% similarities in 2007. Bregmaceros spp. contributed the most within-group similarities in 2011 and 2012 as well as 12.84%, 17.72%, and 23.31% in 2008, 2009, and 2010, respectively. These two taxa contributed the most to within-group similarity, both of which were dominant in five of seven cruises. By contrast, although B. pterotum was the second most frequently occurring taxa and abundant in number (Table 1), it contributed little (only once in 2013) to the within-group similarities in the K assemblage. This indicated that the occurrence of B. pterotum was concentrated in certain stations during the cruises. Therefore, we suggested that mesopelagic Diaphus B was most representative of the KBC during winter, followed by Bregmaceros spp., in terms of abundance and distribution.
In the cold M and M2 assemblages in 2007–2013, the average within-group similarities were higher than those in the K1 assemblage (Table 2). The larval fish structure varied dramatically among years—from dominance by demersal taxa (Triglidae and Callionymidae in 2007, Scorpaenidae in 2008 and 2010, and Gobiidae in 2012 and 2013) to that by mesopelagic taxa (B. pterotum in 2009 and Bregmaceros spp. in 2013) and by pelagic E. japonicus in 2011. In addition, the contributing taxa showed more variations over the years, where Scorpaenidae, Trichiurus spp., and E. japonicas dominated only two of the seven cruises (Table 2). Furthermore, the other contributing taxa only showed single appearances during the cruises. The most contributing taxa in the MCCW was represented by a single taxon in M and M2 in 2007, M in 2011, and M2 in 2013, which implied a higher chance of specific taxa thriving abundantly in the MCCW during winter. Among the numerically abundant B. pterotum, Scorpaenidae, and Gobiidae in M and M2 assemblages (Table 1), Gobiidae showed the most contribution to the high within-group similarity in 2010, 2012, and 2013 (Table 2). Scorpaenidae also contributed the most to within-group similarity in 2008 and 2010. However, no specific larval fish taxon contributed to the within-group similarity persistently throughout the cruises. Therefore, demersal Gobiidae and Scorpaenidae were regarded as the most representative taxa in the MCCW during winter, in terms of abundance and distribution.
Larval fish distribution in relation to hydrographic conditions
The frontal areas among water masses are illustrated in Fig. 5. Overall, larval fish abundance was lower in the MCCW. Moreover, ichthyoplankton was absent in certain stations under the influence of the MCCW during 2007–2013. In 2007, the MCCW intruded at stations 40, 41, and 43 in the middle of the TS and formed a frontal zone with the KBC. In the northern TS, the MCCW reached stations 46, 50, and 52 and collided with the KBCNT. Larval fish abundance was prominently different between water masses, and lower abundance was observed in the MCCW stations. Moreover, the frontal zones served as a barrier for the distribution of ichthyoplankton assemblages, where M and M2 assemblages were confined to the cold MCCW. In 2008, the MCCW retreated to stations 46, 47, and 49 in the northern TS, and the northern boundary reached stations 53 and 50, impinged with the KBCNT in northern Taiwan. The distribution of the M assemblage reached the north of the TS, regardless of the frontal area. By contrast, the K assemblage, dominated by mesopelagic fishes, was confined to the warmer KBC in the middle TS. The position of the MCCW in 2009 was similar to that in 2008. The M assemblage reached the southern TS and was distributed across the frontal area in the middle of the TS. In 2010, the MCCW occupied the northern TS, similar to that in 2009. The geographical distribution of the assemblages generally conformed to the frontal areas. In 2011, the frontal zone was located in the middle of the TS, and the distribution of larval fish assemblage conformed to water mass patterns. In 2012, the MCCW was the strongest and reached its most southernmost point at stations 37 and 38 in the TS; the northern edge of the MCCW was also distributed more toward the south at stations 50 and 52. In addition, the M assemblage was distributed across the frontal area in the northern TS. In 2013, the MCCW was strong and intruded on the southern TS. M and M2 assemblages were confined to the MCCW, and the K assemblage was distributed across the frontal area in the middle of the TS.
Spatial distributions of larval abundance for corresponding groups determined through cluster analyses in the TS during the winters of 2007–2013. The gray line indicates the boundary of water masses derived from the T–S diagram (Kuroshio Front in the north and Peng-Chang Front in the south). K Kuroshio Branch Current assemblage, K2 Kuroshio Branch Current of Northern Taiwan assemblage, M Mixed China Coastal Water assemblage, M2 Mixed China Coastal Water assemblage 2
The BIO-ENV procedure was used to identify the factors with the greatest influence on the larval fish assemblages. In general, the results indicated that the distribution pattern of larval fish assemblages was moderately associated with the environmental factors (Table 3), and the combination of temperature and salinity at 5 m best explained the observed biological patterns. The σw value for each cruise ranged from 0.298 (in 2012) to 0.496 (in 2008). The single environmental variable having the greatest influence on the fish assemblage distribution differed among years. Salinity at 5 m best described the biota pattern in 2007, 2009, 2010, 2012, and 2013, and temperature at 5 m best described the biota pattern in 2008 and 2011 (Table 3).
Discussion
Interannual variations in larval fish assemblage structure
In this study, the dominant larval fish varied considerably among years for assemblages under the influence of the cold MCCW. In February 2003 and 2004, the most abundant taxon under the influence of the cold CCC in the TS was E. japonicus, followed by Scomber spp., Bleekeria mitsukurii, and B. pterotum (Hsieh et al. 2012). E. japonicus also contributed the most to within-group similarity. However, these findings are partially inconsistent with those of the current study. Similarly, although larval fish assemblage under the influence of the KBC showed relatively stable composition in this study, the result differed from that previously described (Online Resource, Table S1). The most representative taxa in the warm KBC in the southern TS during winter were S. gracilis and V. nimbaria, followed by Diaphus spp. (Hsieh et al. 2011, 2012). However, S. gracilis occurred as dominant taxa only in 2011–2012 (Table 1) and was relatively scarce or distributed sparsely in other years. Instead, Diaphus spp., Bregamaceros spp., and B. pterotum were most representative of the KBC in this study. S. gracilis larvae were reported the most abundant larvae in the KC south of Japan, and the spawning months were estimated to be primarily from September to April (Sassa et al. 2004). Diaphus kuroshio possibly spawns throughout the year in the Kuroshio waters. In addition, S. gracilis was reported centered and most abundant in Kuroshio waters east of Taiwan (Hsieh et al. 2007). Furthermore, S. gracilis was the most abundant taxon in the KC east of Taiwan (Chen et al. 2016). Comparing the SST distribution in Hsieh et al. (2012) and this study, the warm KBC in late winter reached almost 25°N in the northern TS in Hsieh et al. (2012), which indicated a much stronger intrusion of the KC to southern TS than that in this study. Therefore, the distribution of S. gracilis in the TS during winter might relate to the strength of KBC intrusion to the TS. The findings also indicated the importance of long-term observations in ichthyoplankton ecology-related studies.
The groupings of larval fish assemblages were defined from cluster analysis individually for each year, at similarity levels of 9–20% during the 7-year surveys (Fig. 4). Instead, if we cut the similarity at universal level in the dendrogram for all years, it might lead to ecologically uninterpretable results (too many or too few groups). Sometimes the clusters in the dendrogram in one year were more compact than the other, possibly due to the differences in intrinsic property of larval fish composition among years. For instance, the clusters in 2009 were more compact than that in 2007 (Fig. 4). Therefore, MDS was conducted to examine the relationship on a two-dimensional plane. Superimposition of the clusters at various levels of similarity on an ordination plot allows us to examine the relationship between groups, although the choice of similarity values is arbitrary. However, viewing clustering and ordination in combination provides us effective measure of checking the adequacy and mutual consistency of both representations. In this study, the stress value for each year is low (Fig. 4; ranging from 0.01 to 0.12), giving confidence that the MDS plots are an accurate representation of the station relationships. Similar situations in the cutting of similarity were found in previous studies (Zhang et al. 2018; Beaver et al. 2019; Nandy and Mandal 2020).
Linkage between assemblage structure and fronts
In this study, the boundaries between water masses in the TS exhibited spatial and temporal geographical variations depending on the extent of MCCW and KBC penetration. Consequently, the fluctuation in the strength of cold water masses resulted in the shifting of frontal area locations and differences in biological or chemical properties among water masses. In addition, the within-group similarity was generally lower in the warm KBC assemblage than in the cold MCCW assemblage. The KC provides a relatively warm and stable environment, suitable for numerous mesopelagic fishes and their larvae and the ecological characteristics, which include low abundance, low biomass, and high biodiversity (Sassa and Konishi 2015). By contrast, hydrographic conditions in the cold CCC and MCCW regions are more complex and unstable, and only certain larval fishes can survive these situations; this resulted in higher variations of the dominant taxa in the MCCW assemblage. Studies have reported that the assemblages in the cold CCC and MCCW are dominated by the larvae of coastal and demersal fishes with lower abundance and species diversity than the warm KC assemblage (Sassa and Konishi 2015; Chen et al. 2016). As a result, we found that the composition of larval fish was more complex in the KBC than that in the MCCW during winter.
The differences in larval fish abundance between the frontal zones were evident. Furthermore, the distribution patterns of larval fish assemblages generally coincided well with the location of the frontal zones (Fig. 5). We observed that the KBC assemblage in the TS was mostly confined to the frontal areas and restricted in the warm KBC waters through the cruises, except for the 2013 cruise. By contrast, the distribution patterns mismatched between the MCCW assemblage and frontal zones to a certain degree in 2008, 2009, 2010, and 2012. In the matching years of 2007, 2011, and 2013, the taxa contributing to the MCCW assemblage mostly belonged to the pelagic family (where E. japonicus contributed to most of the similarities in 2011 and 2013). Regarding the mismatching years, the MCCW assemblage was distributed across the frontal areas, and the contribution taxa mostly belonged to the demersal and benthopelagic families, including Scorpaenidae, Gobiidae, and Trichiurus spp. (Table 2). Therefore, the frontal area between water masses possibly served as sharp boundaries for the distribution of the KBC assemblage, whereas the barrier effect of the frontal area was relatively low for the MCCW assemblage, particularly when demersal taxa dominated.
Wang et al. (2018) observed that the SST front and upwelling in the northeastern waters of Taiwan were shifted seaward or shoreward with the recession or intrusion of the KF during the winters, and the KF in winter may act as the biological barrier of oceanic organisms and larval fishes. Similarly, Wang et al. (2013), a study conducted in a transect in the northern waters of Taiwan during the winter over 4 years, described spatial and temporal variation of larval fish composition among stations related to the dynamics of KF. The abundance of larval fish was usually lower in the nearshore region from the KF, but higher at the offshore stations. A similar phenomenon was found in this study, where the location of the KF varied annually in the northern waters of Taiwan (Fig. 5). The KF is formed in the northern waters of Taiwan by the interaction of the KC shoreward intrusions and the MCCW that flows from the Taiwan Strait. As mentioned, the frontal area possibly served as sharp boundaries for the KBC (K and K2) assemblage, whereas the effect was relatively low for the MCCW assemblage, particularly when demersal taxa dominated. Thus, we observed cross-frontal transport for some demersal species such as Scorpaenidae, Trichiurus spp., and Gobiidae in the study area. However, because of the lack of biological parameters in body length, developmental stage, and age of these fish, explaining the mechanism of cross-frontal transport has been difficult until present.
In this study, B. pterotum and Trichiurus spp. were common in both the KBC and MCCW, regardless of the frontal boundaries. T. lepturus is distributed worldwide in tropical to subtropical waters. It is a benthopelagic species and mostly inhabits the area of the continental shelf in the western Pacific (Nakamura and Parin 1993). Adult T. lepturus species are widely distributed in the ECS and TS with multi-spawning times (Chiou et al. 2006). Moreover, B. pterotum is a mesopelagic lanternfish, which inhabits the waters of the continental shelf, shelf slopes, and near islands. Huang and Chiu (1998) indicated that B. pterotum is widely distributed in the waters around Taiwan, especially in the TS, which corresponds with our results. Therefore, we suggested that these two benthopelagic taxa are less affected by the considerable change in surface temperature and salinity in the TS during winter, which is regarded as a ubiquitous species during winter in the survey area.
Relationships between environmental factors
Although food availability may affect larval survival (Hsieh et al. 2007, 2012), this factor did not significantly explain the ichthyoplankton structure in this study. The distribution patterns of larval fish and zooplankton abundance were not well matched in the northern TS (Fig. 2b, c). Being both the predators on microzooplankton and the prey of zooplanktivorous fish, larval fish represent a crucial trophic linkage in marine food chains (Guan et al. 2017). Studies have suggested at least three basic patterns of relationships between ichthyoplankton and zooplankton abundance: positive, negative, and random (Sanvicente-Añorve et al. 2006). However, using overall zooplankton biomass is likely an approximate measure to reveal any linkages between prey abundance and larval fish survival, because overall zooplankton biomass represents both prey and potential predators for larval fish. In winter, the abundance and diversity of larval fish are the highest in the Kuroshio region than in the shelf region in the southern ECS, regardless of the poor food availability for the larvae (Sassa and Konishi 2015). Similarly, the chlorophyll a and zooplankton biomass are not limiting factors for larval survival during winter in the southern ECS and the waters surrounding Taiwan (Chen et al. 2016). During winter, the abundance of larval fish is positively correlated with the SST in the ECS (Chen et al. 2014). Moreover, Sassa and Konishi (2015) found that SST best explained the larval fish distribution in the ECS during winter. Ichthyoplankton abundance and species diversity increase with increasing temperature and salinity at the shelf break of the ECS during spring (Okazaki and Nakata 2007). Moreover, the larval fish distribution patterns are well defined by the SST, which is representative of the physical oceanographic factors during late winter in the southern ECS (Ichikawa and Chaen 2000). These results matched closely with our findings. Moreover, the faunistic ordination is not one-dimensional, and it would not be expected that a single environmental variable would provide a very successful match in the BIO-ENV procedure. Therefore, the combination of temperature and salinity are considered to be critical factors affecting the larval fish assemblage distribution in the TS during the cold northeasterly monsoon season.
Understanding how the larval fish respond to environmental variability under the changing climate conditions is essential to the management of fishery resources. Although many large marine ecosystems have been the focus of long-term sampling of ichthyoplankton, the mechanism of climate change-induced recruitment variation and ecosystem response are not fully understood (Brodeur et al. 2008). In the northwestern Pacific, a warming and weakening trend of Kuroshio was found during 1993–2013, capable of redistributing mass and energy between the marginal seas and the Pacific (Wang et al. 2016). Furthermore, the interannual variability and decadal variability, including regime shifts for commercially important Japanese common squid, Japanese sardine, Japanese anchovy, and walleye pollock, were associated with the decadal alternation of the warm/cool phases in the Kuroshio/Oyashio ecosystem in Japanese waters (Yatsu et al. 2013). However, the influence of climate change on the ichthyoplankton characteristics in the TS during winter remains unclear in this study (data not shown). In waters at higher latitudes, the fishery resources usually occur in bulk, such as sardine, anchovy, and pollock. By contrast, the lack of a single species that occurs in bulk in the neritic waters in tropical to subtropical waters renders it difficult to construct a quantitative relationship between fish population and climate change. Consequently, the magnitude of variation in ichthyoplankton abundance in relation to climate change is more difficult to detect in tropical to subtropical waters. Furthermore, it is possible that additional unknown factors not included in the analysis contributed to the variability of the larval fish structure in the TS. Therefore, longer time series of sampling will be required to construct models delineating the relationship between environmental factors and larval fish characteristics in the TS.
References
Atwood E, Duffy-Anderson JT, Horne J, Ladd C (2010) Influence of mesoscale eddies on ichthyoplankton assemblages in the Gulf of Alaska. Fish Oceanogr 19:493–507
Beaver JR, Arp CD, Tausz CE, Jones BM, Whitman MS, Renicker TR, Samples EE, Ordosch DM, Scotese KC (2019) Potential shifts in zooplankton community structure in response to changing ice regimes and hydrologic connectivity. Arct Antarct Alp Res 51:327–345
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Brodeur RD, Peterson WT, Auth TD, Soulen HL, Parnel MM, Emerson AA (2008) Abundance and diversity of coastal fish larvae as indicators of recent changes in ocean and climate conditions in the Oregon upwelling zone. Mar Ecol Prog Ser 366:187–202
Busby MS, Duffy-Anderson JT, Mier KL, De Forest LG (2014) Spatial and temporal patterns in summer ichthyoplankton assemblages on the eastern Bering Sea shelf 1996–2007. Fish Oceanogr 23:270–287
Chang Y, Shimada T, Lee MA, Lu HJ, Sakaida F, Kawamura H (2006) Wintertime sea surface temperature fronts in the Taiwan Strait. Geophys Res Lett 33:L23603
Chang Y, Lee MA, Shimada T, Sakaida F, Kawamura H, Chan JW, Lu HJ (2008) Wintertime high-resolution features of sea surface temperature and chlorophyll-a fields associated with oceanic fronts in the southern East China Sea. Int J Remote Sens 29:6249–6261
Chen TA, Wang SL (2006) A salinity front in the southern East China Sea separating the Chinese coastal and Taiwan Strait waters from Kuroshio waters. Cont Shelf Res 26:1636–1653
Chen WY, Lee MA, Lan KW, Gong GC (2014) Distributions and assemblages of larval fish in the East China Sea during the northeasterly and southwesterly monsoon seasons of 2008. Biogeosciences 11:547–561
Chen YK, Chen WY, Wang YC, Lee MA (2016) Winter assemblages of ichthyoplankton in the waters of the East China Sea Shelf and surrounding Taiwan. Fish Sci 82:755–769
Chiou WD, Chen CY, Wang CM, Chen CT (2006) Food and feeding habits of ribbonfish Trichiurus lepturus in coastal waters of south-western Taiwan. Fish Sci 72:373–381
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Gong GC, Chen YLL, Liu KK (1996) Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: implications in nutrient dynamics. Cont Shelf Res 16:1561–1590
Guan L, Dower JF, McKinnell SM, Pepin P, Pakhomov EA, Hunt BPV (2017) Interannual variability in the abundance and composition of spring larval fish assemblages in the Strait of Georgia (British Columbia, Canada) from 2007 to 2010. Fish Oceanogr 26:638–654
Hsieh HY, Lo WT, Liu DC, Hsu PK, Su WC (2007) Winter spatial distribution of fish larvae assemblages relative to the hydrography of the waters surrounding Taiwan. Environ Biol Fishes 78:333–346
Hsieh HY, Lo WT, Wu LJ, Liu DC, Su WC (2011) Comparison of distribution patterns of larval fish assemblages in the Taiwan Strait between northeasterly and southwesterly monsoons. Zool Stud 50:491–505
Hsieh HY, Lo WT, Wu LJ, Liu DC (2012) Larval fish assemblages in the Taiwan Strait, western North Pacific: linking with monsoon-driven mesoscale current system. Fish Oceanogr 21:125–147
Huang JB, Chiu TS (1998) Seasonal and hydrographic variations of ichthyoplankton density and composition in the Kuroshio edge exchange area off northeastern Taiwan. Zool Stud 37:63–73
Ichikawa H, Chaen M (2000) Seasonal variation of heat and freshwater transports by the Kuroshio in the East China Sea. J Mar Syst 24:119–129
Jan S, Sheu DD, Kuo HM (2006) Water mass and throughflow transport variability in the Taiwan Strait. J Geophys Res 111:1–15
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c + c in higher plants, algae 1 2 and natural phytoplankton. Biochemie und Physiologie der Pflanzen 167:191–194
Nakamura I, Parin NV (1993) Snake mackerels and cutlassfishes of the world (families Gempylidae and Trichiuridae). FAO Fish Syno No. 125 15:136
Nandy T, Mandal S (2020) Unravelling the spatio-temporal variation of zooplankton community from the river Matla in the Sundarbans Estuarine System, India. Oceanologia 62:326–346
Norcross BL, Holladay BA, Busby MS, Mier KL (2010) Demersal and larval fish assemblages in the Chukchi Sea. Deep Sea Res Part II 57:57–70
Okazaki Y, Nakata H (2007) Effect of the mesoscale hydrographic features on larval fish distribution across the shelf break of East China Sea. Cont Shelf Res 27:1616–1628
Sanvicente-Añorve L, Soto LA, Espinosa-Fuentes ML, Flores-Coto C (2006) Relationship patterns between ichthyoplankton and zooplankton: a conceptual model. Hydrobiologia 559:11–22
Sassa C, Konishi Y (2015) Late winter larval fish assemblage in the southern East China Sea, with emphasis on spatial relations between mesopelagic and commercial pelagic fish larvae. Cont Shelf Res 108:97–111
Sassa C, Kawaguchi K, Mori K (2004) Late winter larval mesopelagic fish assemblage in the Kuroshio waters of the western North Pacific. Fish Oceanogr 13:121–133
Shepherd FG, Cushing DH (1980) A mechanism for density-dependent survival of larval fish as the basis of a stock–recruitment relationship. ICES J Mar Sci 39:160–167
Wang YC, Chen WY, Chang Y, Lee MA (2013) Ichthyoplankton community associated with oceanic fronts in early winter on the continental shelf of the southern east China sea. J Mar Sci Technol 21:65–76
Wang YL, Wu CR, Chao SU (2016) Warming and weakening trends of the Kuroshio during 1993–2013. Geophys Res Lett 43:9200–9207
Wang YC, Chan JW, Lan YC, Yang WC, Lee MA (2018) Satellite observation of the winter variation of sea surface temperature fronts in relation to the spatial distribution of ichthyoplankton in the continental shelf of the southern East China Sea. Int J Remote Sens 39:4550–4564
Yatsu A, Chiba S, Yamanaka Y, Ito S, Shimizu Y, Kaeriyama M, Watanabe Y (2013) Climate forcing and the Kuroshio/Oyashio ecosystem. ICES J Mar Sci 70:922–933
Zhang W, Mo Y, Yang J, Zhou J, Lin Y, Isabwe A, Zhang J, Gao X, Yu Z (2018) Genetic diversity pattern of microeukaryotic communities and its relationship with the environment based on PCR-DGGE and T-RFLP techniques in Dongshan Bay, southeast China. Cont Shelf Res 164:1–9
Acknowledgements
The authors thank the crew of the Fisheries Researcher I for help with fieldwork and all the participating researchers of the Fisheries Research Institute in the data analysis over the years. We acknowledge Wallace Academic Editing for editing this manuscript. We are grateful to the Council of Agriculture, Taiwan, R.O.C., for the grant (107AS-9.2.2-AI-A1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, YK., Pan, CY., Wang, YC. et al. Interannual variability of larval fish assemblages associated with water masses in winter in the Taiwan Strait during 2007–2013. Fish Sci 87, 131–144 (2021). https://doi.org/10.1007/s12562-020-01489-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01489-z