Abstract
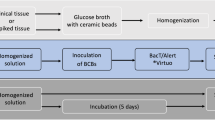
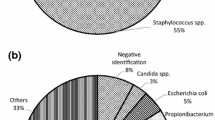
To present our validation study of the BD BACTEC FX blood culture system for sterility testing of cardiovascular tissues aimed for human application. For operational qualification, we performed temperature mapping of the system, vacuum test using non-inoculated BACTEC vials, and growth promotion tests by injecting contaminant strains into aerobic and anaerobic bottles. For performance qualification, negative control, assessment of method suitability, evaluation of sensitivity limits, control of neutralization of antibiotics in biopsy samples from allografts and tissue toxicity effects, were performed. Tissue samples and transport/cryopreservation solutions were homogenized in GentleMACS Dissociator and injected into BACTEC Plus aerobic and anaerobic vials for incubation at 35 °C for 14 days. Tissues were spiked with aerobic and anaerobic bacteria and fungi. Growth of contaminants appeared in all aerobic and anaerobic vials for Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Enterococcus faecalis, Escherichia coli and Pseudomonas aeruginosa; in anaerobic vials for Cutibacterium (Propionibacterium) acnes and Clostridium sporogenes; and only in aerobic vials for Candida albicans and Aspergillus brasiliensis. The majority of bacterial strains were detected within two days (59–100%), exceptionally between 3 and 14 days. In contrast, fungal contaminations were detected within 2, 3–6, 7–10 and after 10 days of incubation in 33.3, 71.6, 96.6 and 99.9% of cases,respectively. Uninhibited growth appeared in the tissue biopsies and homogenized tissues with and without antibiotics and in other solutions. BD BACTEC blood culture system with GentleMACS Dissociator is a rapid and efficient tool for detection of contamination in cardio-vascular tissues aimed for human application.




Similar content being viewed by others
References
Appelbaum PC, Beckwith DG, Dipersio JR et al (1982) Enhanced Detection of Bacteriemia with a new BACTEC Resin Blood Culture Medium. J Clin Microbiol 17(1):48–51
Bossard DA, Ledergerber B, Zingg PO et al (2016) Optimal length of Cultivation Time for Isolation of Propionibacterium acnes in Suspected Bone and Joint Infections Is More than 7 days. J Clin Microbiol 54(12):3043–3049
Buzi M, Guarino A, Gatto C et al (2014) Residual antibiotics in decontaminated human cardiovascular tissue intended for transplantation and risk of falsely negative microbiological analyses. PLoS ONE 9(11):e112679. https://doi.org/10.1371/journal.pone.0112679
Díaz Rodríguez R, Van Hoeck B, Mujaj B et al (2016) Bacteriology testing of cardiovascular tissues: comparison of transport solution versus tissue testing. Cell Tissue Bank 17(2):211–218. https://doi.org/10.1007/s10561-015-9537-2
Dreyer AW (2014) Sepsis—An ongoing and significant challenge blood culture systems: from patient to result chapter 15. In Tech, London
Fan YD, Van Hoeck B, Holovska V, Jashari R (2012) Evaluation of decontamination process of heart valve and artery tissues in European Homograft Bank (EHB): a retrospective study of 1,055 cases. Cell Tissue Bank 13(2):297–304. https://doi.org/10.1007/s10561-011-9255-3
Flahyart D, Borek AP, Wakefield T et al (2007) Comparison of BACTEC PLUS Blood Culture Media to BacT/Alert FA Blood Culture Media for Detection of Bacterial Pathogensin Samples Containing Therapeutic Levels of Antibiotics. J Clin Microbiol 45(3):816–821. https://doi.org/10.1129/JCM.02064-06
Fuller DAD, Davis TE (1997) Comparison of BACTEC Plus Aerobic/F, Anaerobic/F, Pedis Plus/F, and Lytic/F media with and without fastidious organisms supplement to conventional methods for culture of sterile body fluids. Diagn Microbiol Infect Dis 29(4):219–225. https://doi.org/10.1016/S0732-8893(97)00164-8
Funke G, Funke-Kissling P (2004) Use of the BD PHOENIX automated microbiology system for direct identification and susceptibility testing of gram-negative rods from positive blood cultures in a three-phase trial. JCM 42(4):1466–1470
Hensen Suss P, Stadler Tasca Riberio V, Cieslinski, et al (2018) Experimental procedures for decontamination and microbiological testing in cardiovascular tissue banks. Exp Biol Med 243:1284–1299. https://doi.org/10.1177/1535370218820515
Jashari R, Faucon F, Hoeck BV et al (2011) Determination of Residual Antibiotics in Cryopreserved Heart Valve Allografts. Transfus Med Hemother Dec. 38(6):379–386
Jashari R, Goffin Y, Van Hoeck B et al (2010) European homograft bank: twenty years of cardiovascular tissue banking and collaboration with transplant coordination in Europe. Transplant Proc 42(1):183–189. https://doi.org/10.1016/j.transproceed.2009.11.022
Jashari R, Tabaku M, Van Hoeck B et al (2007) Decontamination of heart valve and arterial allografts in the European Homograft Bank (EHB): comparison of two different antibiotic cocktails in low temperature conditions. Cell Tissue Bank 8(4):247–255
Kirihara JM, Hillier SL, Coyle MB (1985) Improved detection Times for Mycobacterium Avium Complex and Mycobacterium tuberculosis with the BACTEC Radiometric System. J Clin Microbiol 22(5):841–845
Minassian AM., Newnham R, Kalimeris E, et al. (2014) Use of automated blood culture system (BD BACTECTM) for diagnosis of prosthetic joint infections: easy and fast. BMC Infectious Disease 14; 233:4–7 http//www.biomedcentral.com/1471-2334/14/233
Palarasah Y, Skjoedt M-O, Lars V et al (2010) Sodium polyanethole sulfonate as an inhibitor of activation of complement function in blood culture systems. J Clin MicrobioL 48(3):908–914. https://doi.org/10.1128/JCM.01985-09
Paolin A, Trojan D, Petit P et al (2017) Evaluation of allograft contamination and decontamination at the treviso tissue bank foundation: a retrospective study of 11,129 tissues. Plos One 7 12(3):e0173154. https://doi.org/10.1371/journal.pone.0173154
Plantamura E, Huyghe G, Panterne B et al (2012) Validation of the BacT/ALERT®3 Dautomated culture system for the detection of microbial contamination of epithelial cell culture medium. Cell Tissue Bank 13:453–459
Schroeter J, Wilkemeyer I, Schiller RA, Pruss A (2012) Validation of the microbiological testing of tissue preparations using the BACTEC TM blood culture system. Transfus Med Hemother 39(6):387–390. https://doi.org/10.1159/000345812
Skenderi Z, Giurgola L, Gatto C et al (2018) Increased sensitivity of microbiological testing of cornea organ culture medium by additional resin treatment. BMJ Open Ophthalmol 10 3(1):e000173. https://doi.org/10.1136/bmjophth-2018-000173
Spaargaren J, Van Boven CPA, Voorn GP (1998) Effectiveness of resins in neutralizing antibiotic activities in bactec plus aerobic/F Culture medium. J Clin Microbiol 36(12):3731–3733
Tabaku M, Jashari R, Carton HF et al (2004) Processing of cardiovascular allografts: effectiveness of European Homograft Bank (EHB) antimicrobial treatment (cool decontamination protocol with low concentration of antibiotics). Cell Tissue Bank 5(4):261–266
Van Kats JP, van Tricht C, van Dijk A et al (2010) Microbiological examination of donated human cardiac tissue in heart valve banking. European J Cardio-Thoracic Surg 37:163–169
Varettas K (2014) Swab or biopsy samples for bioburden testing of allograft musculoskeletal tissue? Cell Tissue Bank 15(4):613–618
Veen MR, Bloem RM, Petit PL (1994) Sinsitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orth Relat Res. 300(300):259–263
Vehmejer SB, Bloem RM, Petit PL (2001) Microbiological screening of of post-mortembone donors – two case reports. J Hosp Infect 47(3):193–197
Zahra S, Galea G, Jashari R, Petit P, de By T (2019) Validation of microbiological testing in ardiovascular tissue establishments: results of a second international quality-round trial. Eur J Clin Microbiol Infect Dis 38:1481–1490. https://doi.org/10.1007/s10096-019-03576-1
Funding
There was no external funding used for this validation paper. As this work was carried out for validation of the system identified to be used for sterility testing of cardio-vascular allografts that are processed by our TE, all costs of the tests were covered by our TE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that there is no conflict of interest for any of them. Dr. Jashar is a Director and Manage of Human Body Material of the EHB.
Ethical approval
The cardio-vascular tissues used for this validation study, were assessed for processing as allografts for clinical application. However, as they were morphologically not suitable for clinical application and subsequently identified for discard, they were used as “validation material” in the study. Informed consent was available for each donor of these tissues.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jashari, R., Vanzeebroeck, S., Petit, P. et al. The BD BACTEC FX blood culture system with the gentlemacs dissociator is suitable for sterility testing of heart valve and vascular allografts—A validation study. Cell Tissue Bank 22, 453–466 (2021). https://doi.org/10.1007/s10561-020-09893-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-020-09893-6