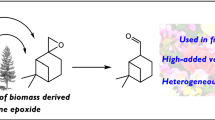
Abstract
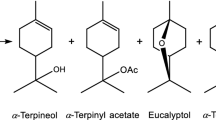
A new type of heterogeneous catalyst for hydration of α-pinene was prepared. Montmorillonite K10 was treated by various acids (H2SO4, HCl, HNO3, and ClCH2COOH) and successfully used for the mentioned reaction. The used characterization techniques showed that the acid treatment improved the properties of K10 important for the catalytic activity (SBET and acidity). On the other hand, the morphology and particle size distribution remained the same. Regarding the selectivity (side and consecutive reactions can proceed), the optimal reaction conditions were found (temperature, type of the catalyst, amount of the catalyst, molar ratio α-pinene: water, type of water, solvent). Using the optimal reaction conditions, 60% conversion of α-pinene was achieved with 45% selectivity to α-terpineol (80 °C, 25 wt% of K10/HCl, or K10/H2SO4, nα-pinene:nwater 1:7.5, 1,4-dioxane as a solvent, 24 h). Higher conversions of α-pinene, as well as higher selectivity to α-terpineol, were achieved using all acid treated K10 in comparison to raw K10. Considering the heterogeneous form of prepared catalysts, its availability, low price and easy method of preparation, these catalysts dispose of a large potential for application as catalysts for hydration reactions.
Graphic Abstract









Similar content being viewed by others
References
Sales A, de Oliveira FL, Bicas JL (2020) Curr. Opin. Food Sci. 37:98
Yadav MK, Patil MV, Jasra RV (2009) J. Mol. Catal. A: Chem. 297:101–109
Avila MC, Comelli NA, Firpo NH, Ponzi EN, Ponzi MI (2008) J. Chil. Chem. Soc. 53(1):1460–1462
Caovilla M, Caovilla A, Pergher SBC, Esmelindro MC, Fernandes C, Dariva C, Bernardo-Gausmao K, Oestreicher EG, Antunes OAC (2008) Catal. Today 133–135:695–698
Tai YN, Xu M, Ren JN, Dong M, Yang ZY, Pan SY, Fan GJ (2016) Sci. Food Agric. 96:954–961
Donaldson ME, Mestre VL, Vinci D, Liotta CL, Eckert CA (2009) Ind. Eng. Chem. Res. 48:2542–2547
Frija LMT, Afonso CAM (2012) Tetrahedron 68:7414–7421
Tsitsishvili V, Ivanova I, Ramishvili T, Kokiashvili N, Bukia T, Dobryakova I, Kurtsikidze G (2017) Bull. Georg. Natl. Acad. Sci. 11(3):79–87
Yahata K, Sakurai S, Hori S, Yoshioka S, Kaneko Y, Hasegawa K, Akai S (2020) Org. Lett. 22:1199–1203
Zukowska K, Paczek L, Grela K (2016) ChemCatChem 8:2817–2823
Comelli N, Avila MC, Volzone C, Ponzi M (2013) Cent. Eur. J. Chem. 11(5):689–697
Prakoso T, Hanley J, Soebianta MN, Soerawidjaja TH, Indarto A (2018) Catal. Lett. 148(2):725–731
Román-Aguirre M, De la Torre-Sáenz L, Flores WA, Robau-Sánchez A, Elguézabal AA (2005) Catal. Today 107:310–314
Pakdel H, Sarron S, Roy C (2001) J. Agric. Food Chem. 49(9):4337–4341
Robles-Dutenhefner PA, da Silva KAH, Siddiqui MR, Kozhevnikov IV, Gusevskaya EV (2001) J. Mol. Catal. A: Chem. 175(1):33–42
Ávila MC, Ponzi MI, Comelli NA (2015) J. Chromatogr. Sep. Tech. 6(7):1–6
Li L, Liu Y, Yu ST, Liu SW, Xie CX, Liu FS (2013) Res. Chem. Intermed. 41(4):2407–2414
Yuan B, Zhong H, Liu P, Xie C, Liu X, Yu F, Yu S, Zhang J (2016) Catal. Lett. 146(5):929–936
Liu SW, Yu ST, Liu FS, Xie CX, Li L, Ji KH (2008) J. Mol. Catal. A: Chem. 279(2):177–181
Vital J, Ramos AM, Silva IF, Valente H, Castanheiro JE (2000) Catal. Today 56(1–3):167–172
Yadav MK, Patil MV, Jasra RV (2009) J. Mol. Catal. A: Chem. 297(2):101–109
van der Waal JC, van Bekkum H, Vital JM (1996) J. Mol. Catal. A: Chem. 105:185–192
Mochida T, Ohnishi R, Horita N, Kamiya Y, Okuhara T (2007) Microporous Mesoporous Mater. 101(1):176–183
Wijayati N, Hidayah N, Mursiti S, Kusumastuti E (2018) IOP Conf. Ser.: Mater. Sci. Eng. 509:012093
Wijayati N, Pranowo HD, Jumina J, Triyono T (2011) Indones. J. Chem. 11(3):234–237
Yang G, Liu Y, Zhou Z, Zhang Z (2011) Chem. Eng. J. 168(1):351–358
Castanheiro JE, Ramos AM, Fonseca I, Vital J (2003) Catal. Today 82(1–4):187–193
Castanheiro JE, Fonseca IM, Ramos AM, Oliveira R, Vital J (2005) Catal. Today 104(2–4):296–304
Vyskočilová E, Gruberová A, Shamzhy M, Vrbková E, Krupka J, Červený L (2018) Reac. Kinet. Mech. Catal. 124(2):711–725
Baishya G, Sarmah B, Hazarika N (2013) Synlett 24(9):1137–1441
Kornatowski J, Baur WH, Pieper G, Rozwadowski M, Schmitz W, Cichowias A (1992) J. Chem. Soc. Faraday Trans. 88(9):1339–1343
R. P. Bell, Dissociation Constants. In Aqueous Solution (2020). https://www.britannica.com/science/acid-base-reaction/Dissociation-constants-in-aqueous-solution. Accessed 23 July 2020
Makarouni D, Lycourghiotis S, Kordouli E, Bourikas K, Kordulis C, Dourtoglou V (2018) Appl. Catal. B 224:740–750
Acknowledgements
This work was realized within the Operational Programme Prague – Competitiveness (CZ.2.16/3.1.00/24501) and “National Program of Sustainability” ((NPU I LO1613) MSMT-43760/2015). This work was also supported from the grant of Specific university research – grant No A2_FCHT_2020_008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sekerová, L., Černá, H., Vyskočilová, E. et al. Preparation of α-Terpineol from Biomass Resource Catalysed by Acid Treated Montmorillonite K10. Catal Lett 151, 2673–2683 (2021). https://doi.org/10.1007/s10562-020-03514-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03514-3