Abstract
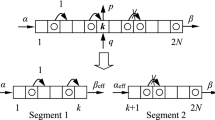
Many cellular processes rely on the cell’s ability to transport material to and from the nucleus. Networks consisting of many microtubules and actin filaments are key to this transport. Recently, the inhibition of intracellular transport has been implicated in neurodegenerative diseases such as Alzheimer’s disease and Amyotrophic Lateral Sclerosis. Furthermore, microtubules may contain so-called defective regions where motor protein velocity is reduced due to accumulation of other motors and microtubule-associated proteins. In this work, we propose a new mathematical model describing the motion of motor proteins on microtubules which incorporate a defective region. We take a mean-field approach derived from a first principle lattice model to study motor protein dynamics and density profiles. In particular, given a set of model parameters we obtain a closed-form expression for the equilibrium density profile along a given microtubule. We then verify the analytic results using mathematical analysis on the discrete model and Monte Carlo simulations. This work will contribute to the fundamental understanding of inhomogeneous microtubules providing insight into microscopic interactions that may result in the onset of neurodegenerative diseases. Our results for inhomogeneous microtubules are consistent with prior work studying the homogeneous case.






Similar content being viewed by others
References
Bressloff PC, Newby JM (2013) Stochastic models of intracellular transport. Rev Mod Phys 85:136–191
Chai Y, Lipowsky R, Klumpp S (2009) Transport by molecular motors in the presence of static defects. J Stat Phys 135:241–260
Dallon JC, Leduc C, Etienne-Manneville S, Portet S (2019) Stochastic modeling reveals how motor protein and filament properties affect intermediate filament transport. J Theor Biol 464:132–148
Gross SP, Vershinin M, Shubeita GT (2007) Cargo transport: two motors are sometimes better than one. Curr Biol 17:R478–R486
Grzeschik H, Harris RJ, Santen L (2010) Traffic of cytoskeletal motors with disordered attachment rates. Phys Rev E 81:031929
Holmes MH (2013) Introduction to perturbation methods, 2nd edn. Texts in Applied Mathematics, 20. Springer, New York, xviii+436 pp
Iniquez A, Allard J (2017) Spatial pattern formation in microtubule post-translational modifications and the tight localization of motor-driven cargo. J Math Biol 74(5):1059–1080
Klumpp S, Lipowsky R (2005) Cooperative cargo transport by several molecular motors. PNAS 102(48):17284–17289
Klumpp S, Nieuwenhuizen TM, Lipowsky R (2005) Self-organized density patterns of molecular motors in arrays of cytoskeletal filaments. Biophys J 88:3118–3132
Klumpp S, Chai Y, Lipowsky R (2008) Effects of the chemomechanical stepping cycle on the traffic of molecular motors. Phys Rev E 78:041909
Kunwar A, Vershinin M, Xu J, Gross JP (2008) Stepping, strain gating, and an unexpected force-velocity curve for multiple-motor-based transport. Curr Biol 18:1173–1183
Lakadamyali M (2014) Navigating the cell: how motors overcome roadblocks and traffic jams to efficiently transport cargo. Phys Chem Chem Phys 16:5907
Liang WH, Li Q, Rifat Faysal KM, King SJ, Gopinathan A, Xu J (2016) Microtubule defects influence kinesin-based transport in vitro. Biophys J 110:2229–2240
Mallik R, Gross SP (2004) Molecular motors: strategies to get along. Curr Biol 14:R971–R982
Müller MJI, Klumpp S, Lipowsky R (2005) Molecular motor traffic in a half-open tube. J Phys Condens Matter 17:S3839–S3850
Newby J, Bressloff PC (2010a) Local synaptic signaling enhances the stochastic transport of motor-driven cargo in neurons. Phys Biol 7:036004
Newby J, Bressloff PC (2010b) Random intermittent search and the tug-of-war model of motor-driven transport. J Stat Mech 4:04014
Parmeggiani A, Franosch T, Frey E (2003) Phase coexistence in driven one-dimensional transport. Phys Rev Lett 90:086601
Parmeggiani A, Franosch T, Frey E (2004) Totally asymmetric simple exclusion process with Langmuir kinetics. Phys Rev E 70:046101
Popkov V, Rákos A, Willmann RD, Kolomeisky AB, Schütz GM (2003) Localization of shocks in driven diffusive systems without particle number conservation. Phys Rev E 67:066117
Rank M, Frey E (2018) Crowding and pausing strongly affect dynamics of kinesin-1 motors along microtubules. Biophys J 115(6):1068–1081
Reese L, Melbinger A, Frey E (2011) Crowding of molecular motors determines microtubule depolymerization. Biophys J 101:2190–2200
Reichenbach T, Frey E, Franosch T (2007) Traffic jams induced by rare switching events in two-lane transport. New J Phys 9:159
Ross JL, Ali MY, Warshaw DM (2008) Cargo transport: molecular motors navigate a complex cytoskeleton. Curr Opin Cell Biol 20:41–47
Rossi LW, Radtke PK, Goldman C (2014) Long-range cargo transport on crowded microtubules: the motor jamming mechanism. Phys A 401:319–329
Seitz A, Kojima H, Oiwa K, Mandelkow E, Song Y-H, Mandelkow E (2002) Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J 21(18):4896–4905
Striebel M, Graf IR, Frey E (2020) A mechanistic view of collective filament motion in active nematic networks. Biophys J 118(2):313–324
Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E (1999) Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci 112(14):2355–2367
Varga V, Leduc C, Bormuth V, Diez S, Howard J (2009) Kinesin-8 motors act cooperatively to mediate length-dependent microtubule depolymerization. Cell 138:1174–1183
Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP (2007) Multiple-motor based transport and its regulation by Tau. Proc Nat Acad Sci 104(1):87–92
Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF (1998) Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92(4):547–557
Acknowledgements
The work of SR was supported by the Cleveland State University Office of Research through a Faculty Research Development Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
A Homogeneous Microtubules: Proof of Theorem 1 and Corollary 1
Equation (17) may be rewritten in the form of a system of two first order ODEs for density \(\rho _\varepsilon \) and flux \(\mathcal {F}_\varepsilon \) (see also (14)):
Next, we discuss the phase portrait for this system with \(\varepsilon \ll 1\), depicted in Fig. 7. Away from curve \(\gamma \) defined by
the trajectories of (47), parametrized by \(0\le x\le \ell \), have almost horizontal slope in \((\rho ,\mathcal {F})\) plane. This is because the slope of \(\rho _\varepsilon \) is of the order \(\varepsilon ^{-1}\), that is \(\rho _\varepsilon '(x)\sim \varepsilon ^{-1}\), whenever the point \((\rho _\varepsilon (x),\mathcal {F}_\varepsilon (x))\) is away from \(\gamma \) (it follows from the first equation in (47)). It would be natural to expect that as \(\varepsilon \) vanishes, trajectory \(\left\{ (\rho _\varepsilon (x),\mathcal {F}_\varepsilon (x)),0\le x \le \ell \right\} \) approaches the arch \(\gamma \) and this trajectory is contained in a given thin neighborhood of \(\gamma \) for sufficiently small \(\varepsilon \). In this subsection, it will be shown that the behavior of the solution is more complicated than simply evolving near \(\gamma \).
To describe how the solution \(\rho _\varepsilon (x)\) behaves for \(\varepsilon \ll 1\), we introduce the following notation for parts of curve \(\gamma \). Namely,
Here \(\rho _\text {eq}:=\varOmega _\mathrm{A}/(\varOmega _\mathrm{A}+\varOmega _\mathrm{D})\). Let us also introduce the following horizontal segment
and the solution g(x; s, a) to (19), i.e., the initial value problem of the first order obtained by the formal limit as \(\varepsilon \rightarrow 0\) in (17):
First, note that \(\gamma _l\), which is the left part of the curve \(\gamma \), is unstable, that is all trajectories, excluding \(\gamma _l\), are directed away from \(\gamma _l\) in the vicinity of \(\gamma _l\). The right part of the curve \(\gamma \), consisting of curve segments \(\gamma _{r,+}\) and \(\gamma _{r,-}\), is stable, attracting all trajectories in its vicinity, except those that follow \(\varGamma \). We note that this exception, when \(\gamma _{r,+}\) loses its stability, occurs at the interface point \((\rho =1/2,\mathcal {F}=\omega _0/4)\) where \(\gamma _{r,+}\) meets \(\gamma _l\). All trajectories reaching this point near (not necessarily intersecting) the curves \(\gamma _{r,+}\) and \(\gamma _l\) continue along \(\varGamma \).
Given specific values of \(\alpha ,\beta \in (0,1)\) in boundary conditions (18), the statement of Theorem 1 as well as representation formula (23) can be simply verified by careful inspection of the phase portrait depicted in Fig. 7. Specifically, for all \(0<\alpha ,\beta <1\), one can draw a path \(\left\{ (\rho (x),\mathcal {F}(x)):0\le x\le \ell \right\} \) along arrows in Fig. 7 (right), which starts at vertical line \(\rho =\alpha \) and ends at vertical line \(\rho =1-\beta \), and such a path will be unique for given \(\alpha \) and \(\beta \) (see also left column of Fig. 8 for specific examples). Instead of checking each couple \((\alpha ,\beta )\), one would split ranges of \((\alpha ,\beta )\) into sub-domains within which the outer solution has constant or smoothly varying shape, as it is done in proof below.
Proof of Theorem 1
Consider the following functions:
These functions can be thought of as one-sided solutions (i.e., satisfying one of the boundary conditions, either \(\rho (0)=\alpha \) or \(\rho (\ell )=\max \left\{ 0.5,1-\beta \right\} \)) of Equation (17) for \(\varepsilon =0\). The reason we choose \(\rho (\ell )=\max \left\{ 0.5,1-\beta \right\} \) instead of \(\rho _\beta (\ell )=1-\beta \) is because there is no solution continuous at \(x=\ell \) with \(\rho (\ell )<0.5\) as visible in Fig. 7 (curve \(\gamma \) is unstable in region \(\left\{ 0\le \rho < 0.5 \right\} \)).
Introduce also the corresponding fluxes:
From the definition of function g it follows that \(\mathcal {F}_\alpha (x)\) and \(\mathcal {F}_\beta (x)\) are both monotonic functions, and function \(\mathcal {F}_\beta (x)\) is defined for all \(0\le x < \ell \). Moreover, \(\mathcal {F}_\beta (x)\) can be extended onto \((-\infty ,\ell ]\) and
Consider case \(\alpha \ge 0.5\). From Fig. 7, it follows that a trajectory emanating for initial point \((\alpha ,\mathcal {F})\) for any \(0<\mathcal {F}<\omega _0/4\) immediately reaches \(\gamma _r\) and stays on \(\gamma _r\cup \varGamma \) for \(0<x\le \ell \). Thus, at \(x=0\) trajectory \(\left\{ (\rho _0(x),\mathcal {F}_0(x)): 0\le x \le \ell \right\} \), describing the outer solution, jumps from \((\alpha ,\mathcal {F}_0(0))\) at \(t=0\) to \(\gamma _r\):
In the case where \(\alpha < 0.5\), denote by \(0\le x_J\le \ell \) location at which fluxes \(\mathcal {F}_\alpha (x)\) and \(\mathcal {F}_\beta (x)\) intersect, that is,
Equality (51) implies that either \(\rho _\alpha (x_J)=1-\rho _\beta (x_J)\) or \(\rho _\alpha (x_J)=\rho _\beta (x_J)\). If \(\rho _\alpha (x_J)=\rho _\beta (x_J)\), then since \(\rho _\alpha \) and \(\rho _\beta \) are solutions of the same first order ordinary differential equation, these two functions coincide \(\rho _\alpha (x)\equiv \rho _\beta (x)\).
We show now that either
Indeed, since \(\alpha <0.5\), trajectory \((\rho _\alpha (x),\mathcal {F}_\alpha (x))\) evolves on \(\gamma _{l}\) for all \(0\le x \le \ell \) where solution \(\rho _\alpha (x)\) exists, and \(\mathcal {F}_{\alpha }(x)\) monotonically increases. Trajectory \((\rho _\beta (x),\mathcal {F}_\beta (x))\) evolves also for all \(0\le x \le \ell \) within either \(\gamma _{r,+}\) or \(\gamma _{r,-}\). If \((\rho _\beta (x),\mathcal {F}_\beta (x))\) evolves within \(\gamma _{r,-}\), then \(\mathcal {F}_\beta (x)\) is monotonically decreasing in x whereas \(\mathcal {F}_\alpha (x)\) is monotonically increasing x, and thus equation \(\mathcal {F}_\alpha (x)=\mathcal {F}_\beta (x)\) can have at most one root in this case. If \((\rho _\beta (x),\mathcal {F}_\beta (x))\) evolves within \(\gamma _{r,+}\), then both \(\mathcal {F}_\alpha (x)\) and \(\mathcal {F}_\beta (x)\) increase with x. Assume that there are at least two distinct numbers \(x_J^{(1)}\), \(x_J^{(2)}\) such that \(x_J^{(1)}<x_J^{(2)}\) and \(\mathcal {F}_\alpha (x_J^{(i)})=\mathcal {F}_{\beta }(x_J^{(i)})\), \(i=1,2\). Assume also that \(x_J^{(1)}\) and \(x_J^{(2)}\) are neighbor roots of equation \(\mathcal {F}_\alpha (x)= \mathcal {F}_{\beta }(x)\), i.e., for all \(x\in (x_J^{(1)},x_J^{(2)})\) we have \(\mathcal {F}_\alpha (x)\ne \mathcal {F}_{\beta }(x)\). Then due to
and \(\rho _\alpha (x_J^{(i)})<0.5\) \(\rho _\alpha (x_J^{(i)})>0.5\), \(i=1,2\), we have that \(\partial _x \mathcal {F}_\alpha (x_J^{(i)})>\partial _x \mathcal {F}_\beta (x_J^{(i)})\), \(i=1,2\). Noting that a smooth function can’t have the same sign of its derivative at two successive roots we arrive to contradiction. Therefore, such \(x_J\) is at most one and (52) is shown.
Left: the thick line represents the trajectories from Examples 1–4; it starts at \(\rho =\alpha \) and ends at \(\rho =1-\beta \), the black circle at (0.8,0.16) represents the stationary solution. Right: The thick line represents the outer solution \(\rho _0(x)\) for Examples 1-4. In Examples 2 and 3, branches \(g(x;0,\alpha )\) and \(g(x;1,\max \{0.5,1-\beta \})\) extend slightly beyond the intervals where they are a part of the outer solution \(\rho _0(x)\) (thin curves)
If \(\mathcal {F}_\alpha (x)\ne \mathcal {F}_\beta (x)\) for all \(0\le x \le 1\), then define \(x_J\) as follows:
We note that point \(x=x_J\) is where the outer solution jumps from \(\rho _\alpha (x)\) to \(\rho _\beta (x)\), thus
and \(\rho _0(\ell )=1-\beta \).
Formulas (50), (53), and (18) complete the proof of Theorem 1. \(\square \)
B Examples of Solutions Given by (23)
To illustrate the result of Theorem 1 we continue with the following examples. We take \(\omega _0=1\), \(\ell =1\), \(\varOmega _\mathrm{A}=0.8\) and \(\varOmega _\mathrm{D}=0.2\), and we vary the boundary rates \(\alpha \) and \(\beta \). The outer solution for each example, as both a trajectory in \((\rho , \mathcal {F})\) plane and the plot of \(\rho _0(x)\), is depicted in Fig. 8.
Example 1
\(\alpha =0.4\) and \(\beta =0.39\).
Example 2
\(\alpha =0.1\) and \(\beta =0.4\).
Example 3
\(\alpha =0.1\) and \(\beta =0.85\).
Example 4
\(\alpha =0.9\) and \(\beta =0.8\).
The case \(x_J>1\) corresponds to the case of fast motor proteins or, more precisely, unidirectional motion dominates attachment/detachment, and thus resulting density is low in MT, \(\rho _0(x)<0.5\) for \(x\in (0,1)\). Consider the following example:
Example 5
\(\alpha =0.05\), \(\beta =0.85\), \(\varOmega _\mathrm{A}=0.16\) and \(\varOmega _\mathrm{D}=0.04\).
The solution is depicted in Fig. 9.
Rights and permissions
About this article
Cite this article
Ryan, S.D., McCarthy, Z. & Potomkin, M. Motor Protein Transport Along Inhomogeneous Microtubules. Bull Math Biol 83, 9 (2021). https://doi.org/10.1007/s11538-020-00838-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00838-4