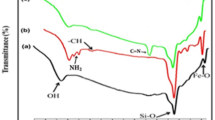
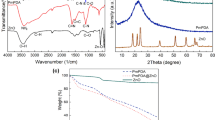
Abstract
Fe2O3–Dy2O3 (FD) was synthesized via green approach using Syzygium aromaticum (Clove) bud extract, for the effective removal of an antibiotic ciprofloxacin. The formation of Fe2O3–Dy2O3 nanocomposite was confirmed by FTIR, XRD, XPS and EDS. A magnetic counterpart c-Fe2O3–Dy2O3 (c-FD) was obtained via calcinations of FD at 700 °C and tested for the CIP removal performance. BET surface area of the FD and c-FD was found to be 112 and 41 m2 g−1. Antibiotics have been emerged as an issue high concern due to their potential risk for ecosystem and human health. There is a crucial need for the development of efficient materials for the recovery of these pollutants from waste water. Aiming on this issue, the synthesized systems were tested for Ciprofloxacin removal. The maximum adsorption capacity of 125 and 328 mg g−1, for both FD and c-FD was obtained, respectively. The maximum percentage removal of CIP was found to be 70% for FD and 53% for c-FD at the adsorbent dose of 1 g L−1 within 90 min for 40 ppm initial concentration of CIP. The effect of FD and c-FD nanocomposites on bacterial strains reveals their specific effect on Gram-negative bacteria, Escherichia coli (E. coli). Further, the regeneration and recyclability of nanoadsorbents showed excellent cycling stability and recyclability given their robustness, which is advantageous for further application in water purification and treatment.
Graphic abstract









Similar content being viewed by others
References
Kummerer, K.: Antibiotics in the aquatic environment—a review—part I. Chemosphere 75, 417–434 (2009)
Bilal, M., Mehmood, S., Rasheed, T., Iqbal, H.M.: Antibiotics traces in the aquatic environment: persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 13, 68–74 (2020)
Bilal, M., Rasheed, T., Mehmood, S., Tang, H., Ferreira, L.F.R., Bharagava, R.N., Iqbal, H.M.: Mitigation of environmentally-related hazardous pollutants from water matrices using nanocomposite d materials—a review. Chemosphere 253, 126770 (2020)
Li, Z., Li, M., Zhang, Z., Li, P., Zang, Y., Liu, X.: Antibiotics in aquatic environments of China: a review and meta-analysis. Ecotoxicol. Environ. Saf. 199, 110668 (2020)
Reygaert, W.C.: An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4(3), 482–501 (2018)
Bilal, M., Ashraf, S.S., Barceló, D., Iqbal, H.M.: Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 691, 1190–1211 (2019)
Correa, E.M.C., Franco, M.F.A., González, C.F.: Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 12, 102–107 (2020)
Tamaddon, F., Nasiri, A., Yazdanpanah, G.: Photocatalytic degradation of ciprofloxacin using CuFe2O4@ methyl cellulose based magnetic nanobiocomposite. Methods X 7, 100764 (2020)
Yu, F., Sun, S., Han, S., Zheng, J., Mab, J.: Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem Eng J. 285, 588–595 (2016)
Taheran, M., Naghdi, M., Brar, S.K., Knystautas, E.J., Verma, M., Surampalli, R.Y.: Degradation of chlortetracycline using immobilized laccase on Polyacrylonitrile biochar composite nanofibrous membrane. Sci. Total Environ. 605, 315–321 (2017)
Carrasquillo, A.J., Bruland, G.L., MacKay, A.A., Vasudevan, D.: Sorption of ciprofloxacin and oxytetracycline zwitterions to soils and soil minerals: influence of compound structure. Environ. Sci. Technol. 42, 7634–7642 (2008)
Wang, C.J., Li, Z.H., Jiang, W.T.: Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl. Clay Sci. 53, 723–728 (2011)
Gu, C., Karthikeyan, K.G.: Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides. Environ. Sci. Technol. 39, 9166–9173 (2005)
Goyne, K.W., Chorover, J., Kubicki, J.D., Zimmerman, A.R., Brantley, S.L.: Sorption of the antibiotic ofloxacin to mesoporous and nonporous alumina and silica. J. Colloid Interface Sci. 283, 160–170 (2005)
Rasheed, T., Bilal, M., Hassan, A.A., Nabeel, F., Bharagava, R.N., Ferreira, L.F.R., Iqbal, H.M.: Environmental threatening concern and efficient removal of pharmaceutically active compounds using metal-organic frameworks as adsorbents. Environ. Res. 184, 109436 (2020)
Haldar, D.J., Duarah, P., Purkait, M.K.: MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: a review. Chemosphere 251, 126388 (2020)
Liu, X.Y., Wang, K.X., Chen, J.S.: Template-directed metal oxides for electrochemical energy storage. Energy Storage Mater. 3, 1–17 (2016)
Obermayer, D., Balu, A.M., Romero, A.A., Goessler, W., Luque, R., Kappe, C.O.: Nanocatalysis in continuous flow: supported iron oxide nanoparticles for the heterogeneous aerobic oxidation of benzyl alcohol. Green Chem. 15, 1530–1537 (2013)
Shelke, S.N., Bankar, S.R., Mhaske, G.R., Kadam, S.S., Murade, D.K., Bhorkade, S.B., Rathi, A.K., Bundaleski, N., Teodoro, O.M.N.D., Zboril, R., Varma, R.S., Gawande, M.B.: Iron oxide-supported copper oxide nanoparticles (Nanocat-Fe-CuO): magnetically recyclable catalysts for the synthesis of pyrazole derivatives, 4-methoxyaniline, and ullmann-type condensation reactions. ACS Sustain. Chem. Eng. 2, 1699–1706 (2014)
Ardakani, M.M., Maleki, M., Khoshroo, A.: High performance electrochemical sensor based on electro deposited iron oxide nanoparticle: catecholamine as analytical probe. J. Iran Chem. Soc. 14, 1659–1664 (2017)
Wu, J., Wang, J., Li, H., Du, Y., Huang, K., Liu, B.: Designed synthesis of hematite-based nanosorbents for dye removal. J. Mater. Chem. A. 1, 9837–9847 (2013)
Lee, Y., Gunten, U.V.: Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 44, 555–566 (2010)
Peings, V., Pigot, T., Baylere, P., Sotiropoulos, J.M., Frayret, J.: Removal of pharmaceuticals by a potassium ferrate(vi) material: from practical implementation to reactivity prediction. Environ. Sci. Water Res. Technol. 3, 699–709 (2017)
Félix, A.M.H., Flores, C.A., Cruz, A.M., Barandiarán, J.M., Guzmán, S.S., Silva, R.C.: Removal of tetracycline pollutants by adsorption and magnetic separation using reduced graphene oxide decorated with α-Fe2O3 nanoparticles. Nanomaterials 9(3), 313 (2019)
Cao, Z., Liu, X., Xu, J., Zhang, J., Yang, Y., Zhou, J., Xu, X., Lowry, G.V.: Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron. Environ. Sci. Technol. 51, 11269–11277 (2017)
Li, X., Xu, H., Chen, Z.S., Chen, G.: Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 270974, 1–16 (2011)
Singh, J., Dutta, T., Kim, K.H., Rawat, M., Samddar, P., Kumar, P.: Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol. 16, 84–108 (2018)
Mondal, P., Purkait, M.K.: Preparation and characterization of novel green synthesized iron–aluminum nanocomposite and studying its efficiency in fluoride removal. Chemosphere 235, 391–402 (2019)
Ahmmad, B., Leonard, K., Islam, M.S., Kurawaki, J., Muruganandham, M., Ohkubo, T., Kurod, Y.: Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv. Powder Technol. 24, 160–167 (2013)
Su, H., Wang, X., Sun, Y., Xu, D., Li, L., Liu, C., Zeng, S., Sun, D.: Enhancing the adsorption capacity of hematite by manganese doping: facile synthesis and its application in the removal of congo red. Bull. Korean Chem. Soc. 38, 1155–1162 (2017)
Mondal, P., Anweshan, A., Purkait, M.K.: Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: a review. Chemosphere 259, 127509 (2020)
Liang, X., Zhu, S., Zhong, Y., Zhu, J., Yuan, P., He, H., Zhang, J.: The remarkable effect of vanadium doping on the adsorption and catalytic activity of magnetite in the decolorization of methylene blue. Appl. Catal. B Environ. 97, 151–159 (2010)
Lemine, O.M., Ghiloufi, I., Bououdina, M., Khezami, L., Mhmed, M.O., Hassan, A.T.: Nanocrystalline Ni doped α-Fe2O3 for adsorption of metals from aqueous solution. J Alloys Compd. 588, 592–595 (2014)
Kumar, V., Jain, A., Wadhawan, S., Mehta, S.K.: Synthesis of biosurfactant coated magnesium oxide nanoparticles for methylene blue removal and selective Pb2+. Sens. IET Nanobiotechnol. 2(3), 4 (2018)
Vanaja, M., Gnanajobitha, G., Paulkumar, K., Kumar, S.R., Malarkodi, C., Annadurai, G.: Phytosynthesis of silver nanoparticles by Cissusquadrangularis: infuence of physicochemical factors. J. Nanostruct. Chem. 3, 1–8 (2013)
Gopinath, K., Chinnadurai, M., Devi, N.P., Bhakyaraj, K., Kumaraguru, S., Baranisri, T., Sudha, A., Zeeshan, M., Arumugam, A., Govindarajan, M., Alharbi, N.S., Kadaikunnan, S., Benelli, G.: One-pot synthesis of dysprosium oxide nano-sheets: antimicrobial potential and cyotoxicity on A549 lung cancer cells. J. Clust. Sci. 28(1), 621–623 (2016)
Deneva, I.M.: Infrared spectroscopy investigation of metallic nanoparticles based on copper, cobalt, and nickel synthesized through borohydride reduction method. J. Chem. Technol. Metall. 45(4), 351–3789 (2010)
Balamurugan, M., Saravanan, S., Soga, T.: Synthesis of iron oxide nanoparticles by using Eucalyptus globulus plant extract. J. Surf. Sci. Nanotech. 12, 363–367 (2011)
Niasari, M.S., Javidi, J.: Synthesis of hollow SiO2 nanoparticles from Dy2O3@SiO2 core-shell nanocomposites via a recyclable sonochemical method. J. Clust. Sci. 23, 1019–1028 (2012)
Kamali, K.Z., Alagarsamy, P., Huang, N.M., Ong, B.H., Lim, H.N.: Hematite nanoparticles-modified electrode based electrochemical sensing platform for dopamine. Sci. World J. 396135, 1–13 (2014)
Nasrabadi, M.R., Pourmortazavi, S.M., Ganjali, M.R., Faridbod, P.N.F., Karim, M.S.: Preparation of dysprosium carbonate and dysprosium oxide efficient photocatalyst nanoparticles through direct carbonation and precursor thermal decomposition. J. Mater. Sci. Mater. Electron. 28, 3325–3336 (2017)
Chandrasekhar, M., Nagabhushana, H., Sudheerkumar, K.H., Dhananjaya, N., Sharma, S.C., Kavyashree, D., Shivakumara, C., Nagabhushana, B.M.: Comparison of structural and luminescence properties of Dy2O3 nanopowders synthesized by co-precipitation and green combustion routes. Mater. Res. Bull. 55, 237–245 (2014)
Ebrahiminezhad, A., Hoseinabadi, Z., Berenjian, A., Ghasemi, Y.: Green synthesis and characterization of zerovalent iron nanoparticles using stinging nettle (Urticadioica) leaf extract. Green Process Synth. 6(5), 1 (2017)
Njagi, E.C., Huang, H., Stafford, L., Genuino, H., Galindo, H.M., Collins, J.B., Hoag, G.E., Suib, S.L.: Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 27(1), 264–271 (2010)
Litter, M.I., Navio, J.A.: Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol. A. 98, 171–181 (1996)
Fetisov, A.V., Kozhina, G.A., Estemirova, SKh., Fetisov, V.B., Gulyaeva, R.I.: XPS study of the chemical stability of DyBa2Cu3O6+δ superconductor. Phys. C 508, 62–68 (2015)
Hawn, D.D., DeKoven, B.M.: Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surf. Interface Anal. 10, 63–74 (1987)
Mills, P., Sullivan, J.L.: A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 16, 723–732 (1983)
Muhler, M., Schlӧgl, R., Ertl, G.: The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene 2. Surface chemistry of the active phase. J. Catal. 138, 413–444 (1992)
Wagner, C.D., Zatko, D.A., Raymond, R.H.: Use of the oxygen KLL Auger lines in identification of surface chemical states by electron spectroscopy for chemical analysis. Anal. Chem. 52, 1445–1451 (1980)
Sarma, D.D., Rao, C.N.R.: XPES studies of oxides of second- and third-row transition metals including rare earths. J. Electron Spectrosc. Relat. Phenom. 20, 25–45 (1980)
Mansingh, S., Acharya, R., Martha, S., Parida, K.M.: Pyrochlore Ce2Zr2O7 decorated over rGO: a photocatalyst that proves to be efficient towards the reduction of 4-nitrophenol and degradation of ciprofloxacin under visible light. Phys. Chem. Chem. Phys. 20, 9872–9885 (2018)
Shankar, S.S., Ahmad, A., Sastry, M.: Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 19(6), 1627–1631 (2003)
Justus, J.S., Roy, S.D.D., Raj, A.M.E.: Synthesis and characterization of hematite nanopowders. Mater. Res. Express 3, 105037–105046 (2016)
Karpagavinayagam, P., Vedhi, C.: Green synthesis of iron oxide nanoparticles using Avicennia marina flower extract. Vacuum 160, 286–292 (2019)
Mansilla, M.V., Zysler, R., Fiorani, D., Suber, L.: Annealing effects on magnetic properties of acicular hematite nanoparticles. Phys. B. 320, 206–209 (2002)
Purnama, B., Wijayanta, A.T., Suharyana: Effect of calcination temperature on structural and magnetic properties in cobalt ferrite nano particles. King Saud Univ. Sci. 31(4), 956–960 (2019)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985)
Shimizu, K., Kubo, T., Satsuma, A., Kamachi, T., Yoshizawa, K.: Surface oxygen atom as a cooperative ligand in Pd nanoparticle catalysis for selective hydration of nitriles to amides in water: experimental and theoretical studies. ACS Catal. 2, 2467–2474 (2012)
Justus, J.S., Roy, S.D.D., Raj, A.M.E.: Synthesis and characterization of hematite nanopowders. Mater. Res. Express. 3, 105037–105046 (2016)
Meng, A., Xing, J., Li, Z., Lian, Q.: Cr-Doped ZnO nanoparticles synthesis, characterization adsorption property and recyclability. ACS Appl. Mater. Interfaces. 7, 27449–27457 (2015)
Muthukumaran, C., Sivakumar, V.M., Thirumarimurugan, M.: Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J. Taiwan Inst. Chem. E. 63, 354–362 (2016)
Yavari, S., Mahmodi, N.M., Teymouri, P., Shahmoradi, N., Maleki, A.: Cobalt ferrite nanoparticles: preparation, characterization and anionic dye removal capability. Taiwan Inst. Chem. Eng. 59, 320–329 (2016)
Kataria, N., Garg, V.K., Jain, M., Kadirvelu, K.: Preparation, characterization and potential use of flower shaped zinc oxide nanoparticles (ZON) for the adsorption of Victoria Blue B dye from aqueous solution. Adv. Powder Technol. 27(4), 1180–1188 (2016)
Weber, W., Morris, J.: Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 89, 31–60 (1963)
Konicki, W., Sibera, D., Mijowska, E., Bielun, Z.L., Narkiewicz, U.: Equilibrium and kinetic studies on acid dye Acid Red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J. Colloid Interface Sci. 398, 152–160 (2013)
Tang, Y., Guo, H., Xiao, L., Yu, S., Gao, N., Wang, Y.: Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf. A Physicochem. Eng. Aspects. 424, 74–80 (2013)
Gao, Y., Li, Y., Zhang, L., Huang, H., Hu, J., Shah, S.M., Su, X.: Adsorption and removal of tetracycline antibiotics form aqueous solution by graphene oxide. J. Colloid Interface Sci. 368, 540–546 (2012)
Fan, H., Ma, X., Zhou, S., Huang, J., Liu, Y., Liu, Y.: Highly efficient removal of heavy metal ions by carboxymethyl cellulose immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohyd. Polym. 213, 39–49 (2019)
Zafar, M.N., Dar, Q., Nawaz, F., Zafar, M., Iqbal, M., Nazar, M.F.: Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 8(1), 713–725 (2019)
Zhang, L., Song, X., Liu, X., Yang, L., Pan, F., Lv, J.: Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 178, 26–33 (2011)
Anirudhan, T.S., Deep, J.R., Nair, A.S.: Fabrication of chemically modified graphene oxide/nano hydroxyapatite composite for adsorption and subsequent photocatalytic degradation of aureomycine hydrochloride. J. Ind. Eng. Chem. 47, 415–430 (2017)
Tabrizian, P., Ma, W., Bakr, A., Rahaman, M.S.: pH-sensitive and magnetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high-efficiency removal of tetracyclines. J. Colloid Interface Sci. 534, 549–562 (2019)
Glu, S.T.D., Bayazit, S.S., Kerkez, O., Alhogbid, B.G., Salam, M.A.: Removal of ciprofloxacin from aqueous solution using humic acid and levulinic acid coated Fe3O4 nanoparticles. Chem. Eng. Res. Des. 123, 259–267 (2017)
Yu, F., Sun, S., Han, S., Zheng, J., Ma, J.: Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 285, 588–595 (2016)
Arakha, M., Pal, S., Samantarrai, D.: Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci Rep 5, 14813 (2015)
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, A., Aashima Sharma, Kapur, A. et al. Hematite dysprosium oxide nanocomposites biosynthesized via greener route for ciprofloxacin removal and antimicrobial activity. J Nanostruct Chem 11, 437–453 (2021). https://doi.org/10.1007/s40097-020-00379-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-020-00379-1