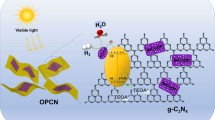
Abstract
Developing an efficient photocatalyst, catalyzing formic acid (FA) dehydrogenation, can satisfy the demand of the H2 energy. Herein, a graphitic carbon nitride (g-CxN4)-based nanosheet (x = 3.2, 3.6 or 3.8) with melem rings conjugated by Schiff-base bond (N=C–C=N) was synthesized, tuning the bandgaps (Eg) of graphitic carbon nitride (g-C3N4) in the range of 1.8 < Eg < 2.7 eV, and grown PdAg nanowires (NWs) on its surface forming an efficient PdAg NWs/g-CxN4 Mott–Schottky heterojunction for enhancing dehydrogenation photocatalysis of FA. The boosting photocatalysis benefits from the Schiff-base bond tuning the Eg of g-C3N4 and strongly coupling from the heterojunction. Among the heterojunction, the Pd5Ag5 NWs/g-C3.6N4 exhibits the best dehydrogenation photocatalysis of FA [turnover frequency (TOF) = 1230 h−1] under visible light (λ > 400 nm) without any additive at 25 °C, which is the best value among ever-reported ones. This work provides a new strategy to boost dehydrogenation photocatalysis of FA, which will be promising for practical application of H2 in future energy field.
Graphical abstract







Similar content being viewed by others
References
Loges B, Boddien A, Gartner F, Junge H, Beller M. Catalytic generation of hydrogen from formic acid and its derivatives: useful hydrogen storage materials. Top Catal. 2010;53(13):902.
Ma ZH, Liu H, Yue M. Magnetically recyclable Sm2Co17/Cu catalyst to chemoselectively reduce the 3-nitrostyrene into 3-vinylaniline under room temperature. Nano Res. 2019;12(12):3085.
Johnson TC, Morris DJ, Wills M. Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem Soc Rev. 2010;39(1):81.
Wang HH, Zhang JF, Chen ZL, Zhang MM, Han XP, Zhong C, Deng YD, Hu WB. Polycrystal Pd nanoparticles: green synthesis and size-dependent performance for formic acid oxidation. Rare Met. 2019;38(2):115.
Zhou H, Wang XH, Liu HZ, Gao SC, Yan M. Improved hydrogen storage properties of LiBH4 confined with activated charcoal by ball milling. Rare Met. 2019;38(4):321.
Zhai TT, Xu S, Yang T, Yuan ZM, Zhang YH. Hydrogen storage properties of La1−xPrxMgNi3.6Co0.4 (x = 0–0.4) alloys with annealing treatment. Rare Met. 2019;38(9):871.
Zhang YH, Gong PF, Li LW, Sun H, Feng DC, Guo SH. Hydrogen storage thermodynamics and dynamics of La–Mg–Ni-based LaMg12-type alloys synthesized by mechanical milling. Rare Met. 2019;38(12):1144.
Wang ZL, Yan JM, Ping Y, Wang HL, Zheng WT, Jiang Q. An efficient CoAuPd/C catalyst for hydrogen generation from formic acid at room temperature. Angew Chem Int Ed. 2013;52(16):4406.
Yu WY, Mullen GM, Flaherty DW, Mullins CB. Selective hydrogen production from formic acid decomposition on Pd–Au bimetallic surfaces. J Am Chem Soc. 2014;136(31):11070.
Zhang S, Metin O, Su D, Sun SH. Monodisperse AgPd alloy nanoparticles and their superior catalysis for the dehydrogenation of formic acid. Angew Chem Int Ed. 2013;52(13):3681.
Wang H, Zhang J, Chen Z, Zhang M, Han P, Zhong C, Deng Y, Hu W. Size-controllable synthesis and high-performance formic acid oxidation of polycrystalline Pd nanoparticles. Rare Met. 2019;38(2):115.
Liu H, Yu YS, Yang WW, Lei WJ, Gao MY, Guo SJ. High-density defects on PdAg nanowire networks as catalytic hot spots for efficient dehydrogenation of formic acid and reduction of nitrate. Nanoscale. 2017;9(27):9305.
Hu C, Mu X, Fan J, Ma H, Zhao X, Chen G, Zheng NF. Interfacial effects in PdAg bimetallic nanosheets for selective dehydrogenation of formic acid. ChemNanoMat. 2016;2(1):28.
Jiang Y, Fan X, Xiao X, Qin T, Zhang L, Jiang F, Chen L. Novel AgPd hollow spheres anchored on graphene as an efficient catalyst for dehydrogenation of formic acid at room temperature. J Mater Chem A. 2016;4(2):657.
Schlapbach L, Zuttel A. Hydrogen-storage materials for mobile applications. Nature. 2001;414(6861):353.
Dutta S, Kim J, Ide Y, Kim JH, Hossain MSA, Bando Y, Yamauchi Y, Wu KCW. 3D network of cellulose-based energy storage devices and related emerging applications. Mater Horizons. 2017;4(4):522.
Gu X, Lu ZH, Jiang HL, Akita K, Xu Q. Synergistic catalysis of metal-organic framework-immobilized Au-Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J Am Chem Soc. 2011;133(31):11822.
Li Y, Yang Y, Huang JW, Wang L, She HD, Zhong JB, Wang QZ. Preparation of CuS/BiVO4 thin film and its efficaciously photoelectrochemical performance in hydrogen generation. Rare Met. 2019;38(5):428.
Sun J, Zhang M, Wang ZF, Chen HY, Chen Y, Murakami N, Ohno T. Synthesis of anatase TiO2 with exposed 001 and 101 facets and photocatalytic activity. Rare Met. 2019;38(4):287.
Yu C, Guo XF, Xi Z, Michelle M, Yin ZY, Shen B, Li JR, Seto C, Sun SH. AgPd nanoparticles deposited on WO2.72 nanorods as an efficient catalyst for one-pot conversion of nitrophenol/nitroacetophenone into benzoxazole/quinazoline. J Am Chem Soc. 2017;139(16):5712.
Bao SY, Liu H, Liu YQ, Yang WW, Wang YJ, Yu YS, Sun YY, Li KF. Amino-functionalized graphene oxide-supported networked Pd-Ag nanowires as highly efficient catalyst for reducing Cr(VI) in industrial effluent by formic acid. Chemosphere. 2020;257:127245.
Liu H, Liu XY, Yang WW, Shen M, Geng S, Yu C, Yu YS. Photocatalytic dehydrogenation of formic acid promoted by a superior PdAg@gC3N4 Mott–Schottky heterojunction. J Mater Chem A. 2019;7(5):2022.
Kumar S, Dhar DN, Saxena PN. Applications of metal complexes of Schiff bases—a review. J Sci Ind Res. 2009;68(3):181.
Jürgens B, Irran E, Senker J, Kroll P, Müller H, Schnick W. Melem (2,5,8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies. J Am Chem Soc. 2003;125(34):10288.
Lau VW, Moudrakovski I, Botari T, Weinberger S, Mesch MB, Duppel V, Senker J, Blum V, Lotsch BV. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat Commun. 2016;7(1):12165.
Li HJ, Sun BW, Sui L, Qian DJ, Chen M. Preparation of water-dispersible porous g-C3N4 with improved photocatalytic activity by chemical oxidation. Phys Chem Chem Phys. 2015;5(17):3309.
Liu H, Guo Y, Yu YS, Yang WW, Shen M, Liu XY, Geng S, Li JR, Yu C, Yin ZY, Li HB. Surface Pd-rich PdAg nanowires as highly efficient catalysts for dehydrogenation of formic acid and subsequent hydrogenation of adiponitrile. J Mater Chem A. 2018;6(36):17323.
Liu H, Liu XY, Yu YS, Yang WW, Li J, Feng M, Li HB. Bifunctional networked Ag/AgPd core/shell nanowires for the highly efficient dehydrogenation of formic acid and subsequent reduction of nitrate and nitrite in water. J Mater Chem A. 2018;6(11):4611.
Liu H, Huang BL, Zhou JH, Wang K, Yu YS, Yang WW, Guo SJ. Enhanced electron transfer and light absorption on imino polymer capped PdAg nanowire networks for efficient room-temperature dehydrogenation of formic acid. J Mater Chem A. 2018;6(5):1979.
Gong Y, Quan X, Yu H, Chen S. Synthesis of Z-scheme Ag2CrO4/Ag/g-C3N4 composite with enhanced visible-light photocatalytic activity for 2, 4-dichlorophenol degradation. Appl Catal B Environ. 2017;219:439.
Acknowledgement
This study was financially supported by Heilongjiang Science Foundation (No. LH2020B006), the National Natural Science Foundation of China (Nos. 51871078, 21871221 and 21602175), the Fundamental Research Funds for the Central Universities (No. 3102017jc01001) and Start-Up Funding for Class D Talent of Xi’an University of Architecture and Technology (No. 1608720038).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Li, XX., Liu, XY. et al. Schiff-base-rich g-CxN4 supported PdAg nanowires as an efficient Mott–Schottky catalyst boosting photocatalytic dehydrogenation of formic acid. Rare Met. 40, 808–816 (2021). https://doi.org/10.1007/s12598-020-01637-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-020-01637-5