Abstract
Rapid eye movement (REM) sleep behavior disorder (RBD) is characterized by dream enactment and the loss of muscle atonia during REM sleep, known as REM sleep without atonia (RSWA). RBD can result in significant injuries, prompting patients to seek medical attention. However, in others, it may present only as non-violent behaviors noted as an incidental finding during polysomnography (PSG). RBD typically occurs in the context of synuclein-based neurodegenerative disorders but can also be seen accompanying brain lesions and be exacerbated by medications, particularly antidepressants. There is also an increasing appreciation regarding isolated or idiopathic RBD (iRBD). Symptomatic treatment of RBD is a priority to prevent injurious complications, with usual choices being melatonin or clonazepam. The discovery that iRBD represents a prodromal stage of incurable synucleinopathies has galvanized the research community into delineating the pathophysiology of RBD and defining biomarkers of neurodegeneration that will facilitate future disease-modifying trials in iRBD. Despite many advances, there has been no progress in available symptomatic or neuroprotective therapies for RBD, with recent negative trials highlighting several challenges that need to be addressed to prepare for definitive therapeutic trials for patients with this disorder. These challenges relate to i) the diagnostic and screening strategies applied to RBD, ii) the limited evidence base for symptomatic therapies, (iii) the existence of possible subtypes of RBD, (iv) the relevance of triggering medications, (v) the absence of objective markers of severity, (vi) the optimal design of disease-modifying trials, and vii) the implications around disclosing the risk of future neurodegeneration in otherwise healthy individuals. Here, we review the current concepts in the therapeutics of RBD as it relates to the above challenges and identify pertinent research questions to be addressed by future work.
Similar content being viewed by others
Overview
Rapid eye movement (REM) sleep behavior disorder (RBD) is an important parasomnia characterized by the loss of skeletal muscle paralysis that normally occurs during REM sleep [1]. Clinically, this motor activity may be perceived as the “acting out of one’s dreams” and can range from violent and potentially injurious behaviors, such as kicking and punching, to more benign purposeful movements such as knitting or piano playing, or identified by the presence of subclinical excess of muscle tone and/or phasic muscle twitching during REM sleep (“REM sleep without atonia,” RSWA) detected by polysomnography (PSG) [2]. RBD is classified as “idiopathic” or “isolated” RBD (iRBD) when it occurs in the absence of a comorbid disorder, or “secondary” and “symptomatic” RBD when it is associated with an underlying condition or following the use of medications, especially antidepressants [3]. The synucleinopathies are most frequently associated with secondary/symptomatic RBD, including Parkinson’s disease (PD; 34–58% of cases) [4,5,6], Dementia with Lewy bodies (DLB; 80–90% of cases) [7, 8] and multiple system atrophy (MSA; 85–100% of cases) [9]. In addition, RBD is also a feature of narcolepsy [10, 11] and is an established symptom of IgLON-5, LGI1, and CASPR2 antibody–associated encephalitides [12,13,14,15]. Furthermore, RBD has also been reported in association with structural lesions of different etiologies [16, 17], as well as in psychiatric conditions such as post-traumatic stress disorder [18]. Rarely, RBD occurs in the setting of non-synuclein neurodegenerative diseases but most likely represents a degree of co-mingled synuclein pathology in these cases [7, 19, 20].
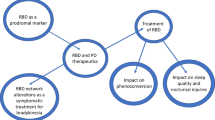
The field of RBD has evolved rapidly since its first description in humans in 1986 [1]. This has been fueled by the exciting discovery that iRBD represents a prodromal stage of synuclein-based neurodegenerative disorders, with 70–90% of patients eventually developing PD, DLB, or MSA [21, 22]. Following this discovery, there have been concerted efforts by the research community to try to understand and halt disease progression in iRBD with much emphasis going toward identifying biomarker profiles of those who are at highest risk of phenoconversion and thus those who may benefit most from future neuroprotective interventions. However, despite progress, there has been an unfortunate paucity of innovation of novel/targeted therapies and of randomized controlled trials focusing on symptomatic or preventative therapies for RBD. Indeed, recent negative findings [23, 24] have exposed our limited understanding of existing symptom management approaches in RBD and have highlighted the need for development of novel therapies for RBD. Moreover, there is still uncertainty surrounding issues relating to optimal trial design, such as a better means of tracking the frequency and severity of injurious movements for symptomatic trials, and an improved understanding of the natural history of RBD. In addition, a refined understanding of the trajectory of phenoconversion will be required to inform optimal patient selection before disease-modifying therapeutic trails can be designed for patients with iRBD.
We review the current literature around the treatment of RBD and highlight outstanding questions that should be addressed in future work to facilitate the design of neuroprotective and symptomatic therapy trials in the coming decade. A systematic search of online databases was performed, including Medline, Embase, and the Cochrane Database of Systematic Reviews, using the search terms “REM Sleep Behavior*” and “Rapid Eye Movement Sleep.” Reference lists of landmark studies were also consulted. We organized the discussion of RBD therapy around the following key areas: diagnosis and screening, optimal symptomatic targets for RBD, the heterogeneity of RBD, the role of medications as triggers of RBD, issues relating to the selection and treatment of patients with isolated RBD, and the disclosure of risk relating to potential future neurodegeneration.
Controversy 1: How Should We Screen and Diagnose RBD?
Accurate and timely identification and diagnosis of RBD is crucial toward minimizing its injurious potential and, ultimately, preventing phenoconversion to a defined neurodegenerative disease phenotype of dementia or parkinsonism. First, this requires increased awareness of RBD by general clinicians and sleep technicians outside of tertiary or specialist facilities [3]. Second, simple and scalable instruments need to be used to screen for and diagnose RBD at a clinic and population level. Third, mimics of RBD including non-REM parasomnias, periodic limb movements of sleep, nocturnal seizures, and psychogenic disorders need to be systematically excluded [4]. Finally, standardized methodology for diagnosing RBD based on objective criteria is required.
Video Polysomnography
Currently, the gold standard method of confirming RBD is video polysomnography, with video demonstration of complex motor behaviors accompanied by RSWA being the diagnostic hallmark of the condition [25, 26]. Recent revisions of the International Classification of Sleep Disorders, third edition (ICSD-3), criteria have emphasized diagnostic thresholds for RSWA that rely on quantification of the amount of excessive muscle activity during REM sleep, for which several well-established and validated visual and automated scoring methods exist [27,28,29,30,31,32]. However, several discrepancies exist between these quantitative scoring techniques. Differences with respect to which muscles are analyzed, criteria for phasic muscle activity (in addition to tonic muscle activity), epoch lengths, amplitude of electromyographic (EMG) activation, and minimum duration of excessive activity will likely result in variations in diagnosis. While various groups have utilized different RSWA scoring approaches, submentalis (chin) RSWA cutoff values for the diagnosis of RBD are quite similar across several studies (approximately 15–20%) [28, 29, 31, 33], suggesting differing definitions for RSWA may yield similar results for diagnostic purposes. Yet, more definitive future studies utilizing a harmonized RSWA scoring method are needed. Cutoff values have been derived from patients with and without clinical RBD [31, 34] and those with concomitant PD [35] and OSA [28, 33] and have been incorporated in the ICSD-3 criteria. The ICSD-3 criteria have proposed utilizing the SINBAR montage cutoff of 27.2% (“any” chin RSWA and phasic flexor digitorum superficialis (FDS) RSWA) for a diagnosis of RBD. However, arm muscles are not routinely recorded in clinical sleep medicine practice when parasomnia is not explicitly anticipated, so the FDS and arm muscle diagnostic thresholds are not universally applicable for RBD diagnosis in patient populations with other sleep comorbidities such as obstructive sleep apnea. Alternatively, the diagnostic cutoffs for any chin RSWA (18.2–21.6%) are highly sensitive (85–100%) and specific (97–100%) for RBD diagnosis across several studies by different groups [25, 31, 36].
Recently, it has become clear that some adult patients without dream enactment behavior history or overt neurological disorders may have also demonstrated excessive levels of RSWA during polysomnography. Isolated RSWA in the absence of dream enactment behaviors could represent an even earlier manifestation of prodromal synucleinopathy, although it remains unclear if such patients are at risk of developing future clinical RBD or other synucleinopathies. One study of 14 patients with isolated RSWA who were followed longitudinally demonstrated that 10 of 14 patients had the presence of neurodegenerative markers, that RSWA increased over time, and that overall 2 of these 14 patients eventually developed RBD [28, 31, 33,34,35, 37]. Clear definitions of “normative” quantitative RSWA values remain a limitation in the field. A recent large study of adult patients without dream enactment behaviors proposed that those exceeding the 95th percentile could be defined as having isolated RSWA [34]. Tonic, phasic, or combined RSWA values have also shown promise toward indicating the presence of an evolving synucleinopathy [38, 39]. Recently, an “any” muscle activity threshold value of 46.4% in the combined submentalis and anterior tibialis muscles was shown to predict a higher rate of phenoconversion to parkinsonism or mild cognitive impairment in a cohort of 60 patients with iRBD [38]. Prospective validation in large-scale multicenter iRBD patient populations will be necessary to determine clinically generalizable RSWA thresholds for prediction of phenoconversion and to determine the prognostic value of RSWA in comparison to other clinical biomarkers.
Aside from differences in thresholds for classifying excessive tonic and phasic movements, protocol variations relating to the particular muscles recorded during polysomnography can also impact sensitivity [36, 40]. Several studies have now shown that addition of upper-extremity electromyography significantly improves the sensitivity for RBD diagnosis; however, rare patients present with movements largely limited to the legs, highlighting the importance of evaluating multiple muscles for RSWA as well as a video review of PSG for abnormal behaviors [31, 40]. More recently, automated and machine learning approaches for RSWA scoring have been published, which have shown promise for more time-efficient and standardized approaches for RBD diagnosis, although these approaches require further validation in large-scale multicenter studies [41, 42]. Standardization of RSWA scoring and polysomnography recording practices to optimize RBD detection will be important for the accurate recruitment of patients and generalization of findings from therapeutic trials in RBD.
The duration of video PSG necessary for accurate RBD detection remains uncertain, with some experts recommending a minimum REM duration of 10% of total sleep time to diagnose RBD, with a second sleep study suggested if the duration of recorded REM sleep is short [43]. However, published studies evaluating RSWA diagnostic thresholds for RBD have typically required at least 5 min of recorded REM sleep for evaluation, so shorter durations may still provide ample diagnostic information in selected cases [28, 31, 33]. There have been a small number of studies [44,45,46] exploring night-to-night variability in manual and automated scoring methods for diagnosing RBD. These studies have shown a reasonable correlation between PSG measures across consecutive nights, with tonic chin muscle activity appearing more stable night-to-night and phasic muscle activity being more variable across nights [44]. Additionally, one study found that PSG alone was detected over 80% of patients with RBD during a single night, but more than 95% of patients met diagnostic criteria for RBD when combined with video analysis [46], suggesting that reliance on RSWA alone (without video analysis) may be inadequate for accurate RBD diagnosis in suspected patient populations.
Automated Scoring Algorithms
Visual/manual scoring of RSWA is time-consuming and requires a trained and experienced interpreter. Automated scoring tools have been developed to quantify excessive REM sleep muscle activity and classify patients with and without RSWA [36, 41, 47,48,49]. These still require optimal technical recordings, and skilled visual/manual removal of epochs with significant artifacts and mimics of RSWA, such as spontaneous or respiratory arousal during REM sleep. Additionally, automatic quantitation tools have been designed to focus on analyzing chin or chin plus arm muscle activity [28, 33, 41, 48], introducing similar limitations as discussed previously in relation to the visual/manual scoring methods. Before well-validated automatic RSWA analysis algorithms become widely available, direct comparisons between different automated methods with rigorous validation across several clinical and ethnic populations is needed.
Questionnaires
Although PSG remains the important gold standard for RBD diagnosis, PSG requires specialized knowledge for appropriate scoring and is not practically applicable to population-wide screening for research or for clinical practice in resource-limited settings. Several RBD screening instruments have been developed and validated in recent years including the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ) [50], the Hong Kong REM Sleep Behavior Disorder Questionnaire (RBDQ-HK) [51], the Mayo Sleep Questionnaire (MSQ) [52], the Innsbruck Sleep Behavior Disorder Inventory (RBD-I) [53], and the single-question RBD1Q [54]. There are several limitations to the use of questionnaires for diagnosing RBD. It is estimated that up to 44% of patients with RBD may not be aware of their dream enactment behaviors so bedpartner input for an accurate diagnosis is paramount [2, 55]. Regarding RSWA, up to 70% of patients go undetected in population screening studies using PSG [56], and conversely, some studies have indicated that isolated RSWA during PSG is common, found in between 14 and 32% of adults without dream enactment symptoms [34, 57]. Most self-administered instruments are also unable to exclude RBD mimics such as OSA, PLMS, and non-REM parasomnias [58, 59], although the MSQ does include screening questions for a broader range of sleep disturbances (freely available from https://www.mayoclinic.org/documents/msq-copyrightfinal-pdf/doc-20079462) [60]. There is also evidence that questionnaires may have different validities depending on the populations studied [61,62,63,64], with poorer performance when translated from clinical sleep medicine to community settings [60, 65, 66]. Furthermore, cutoffs developed for healthy populations on certain questionnaires such as the RBDSQ and HKRBD may not be applicable to patients with neurodegenerative diseases such as PD or DLB, and a higher total RBDSQ cutoff score for RBDSQ is more suitable in PD [67] while the converse has been suggested in DLB [68].
The lack of a unified screening instrument may limit generalization of epidemiological and therapeutic studies, which have recruited patients based on the various available instruments. Consideration of the different psychometric properties of these instruments, enabled by head-to-head comparison studies will be necessary to facilitate an informed decision regarding their use in future trials.
Actigraphy
Overall, the subjective nature of questionnaires imposes limits on the sensitivity and specificity of detecting RBD. Thus, there is a significant need for investigating alternative and more objective screening strategies for RBD that would ideally remain highly feasible, convenient, and inexpensive. Home monitoring and wearable devices represent a promising avenue for RBD screening [69]. One recent study found that wrist actigraphy that was visually interpreted by expert sleep medicine raters was able to discriminate RBD behaviors from other movements in sleep and performed marginally better than questionnaires at identifying iRBD [70]. Further studies exploring the utility of these and other technologies including mobile phone–based applications in identifying RBD will be essential to improving the screening and early identification of asymptomatic iRBD cases for recruitment into therapeutic trials.
Controversy 2: What Is the Optimal Symptomatic Therapy for RBD?
The primary goals of symptomatic management of RBD are to prevent sleep-related injury and minimize the frequency and severity of disturbing nightmares and related complex vocal and motor behaviors. Educating patients newly diagnosed with RBD and their partners about the condition is the most important initial priority, as well as advising on safety modifications including the removal of potentially dangerous items such as sharp-cornered furniture or weapons in the bedroom environment, addition of bed rails, lowering of the mattress closer to the floor, use of a pressure-sensitive bed alarm, and considering separate bedroom quarters for the bedpartner’s safety when the patient’s behaviors have been especially sleep disturbing or dangerous [71,72,73]. Initial symptom management in RBD also includes minimizing or substituting provoking substances (such as antidepressants, alcohol) and timely identification and treatment of any comorbid sleep condition that may also influence the frequency and severity of nocturnal behaviors such as OSA, periodic limb movement disorder, and narcolepsy. Depending on an individual’s frequency and severity of sleep behaviors and their receptiveness to implementing the above measures, pharmacotherapy should also be considered for patients with RBD.
Despite significant advances in our understanding of the biology of RBD, the therapeutic armamentarium for managing this condition has not evolved significantly for the past two decades. With very few randomized controlled trials, the optimal pharmacotherapy for RBD remains an unresolved question in the field. The most used pharmacologic agents are melatonin and clonazepam, with evidence supporting efficacy, safety, and tolerability limited mainly to retrospective or survey-based treatment outcome case series, small trials, and expert consensus. Furthermore, negative findings from recently published randomized placebo-controlled trials using clonazepam [23] and melatonin [24] in RBD have called into question the true efficacy and evidence base of our existing treatments and have spurred the need for innovation to better understand the pathophysiology of dream enactment in RBD, and toward future development of more biologically based approaches to RBD management. Given this, we start this section on pharmacological therapies by briefly reviewing our current understanding of the biology of RBD, thus providing background for the validity and evidence underlying the available symptomatic therapies (Tables 1 and 2) and potential novel approaches discussed further below.
Biology of RBD
Meticulous work in animal models has shown that the glutamatergic neurons of the subcoeruleus (SC, humans)/sublaterodorsal tegmental nucleus (SLD, analogous structure in rodents) located in the dorsolateral pons play a central role in the generation of REM sleep [75]. Moreover, lesions in this region have been linked to RBD in humans [76,77,78]. SC/SLD neurons induce muscle atonia via descending glutamatergic projections to the ventromedial medulla, which, in turn, inhibit the output of spinal motor neurons via glycinergic and GABAergic premotor neurons [79,80,81]. The firing of SC/SLD neurons is tightly regulated by inhibitory GABAergic and glycinergic neurons in the ventrolateral periaqueductal gray (vlPAG) and the adjacent lateral pontine tegmentum (LPT) [82]. However, the vlPAG, LPT, and in some cases the SC/SLD itself, are regulated by a larger network of brainstem and hypothalamic regions operating via a range of neurotransmitters including GABA (preoptic region, ventromedial medulla), orexin and melanin-concentrating hormone (lateral hypothalamus), serotonin (dorsal raphe), and noradrenaline (locus coeruleus) [75]. Experimental findings suggest that the above circuitry is also under circadian control via suprachiasmatic nucleus (SCN; the circadian pacemaker), though the precise pathways for this are not understood [83]. Therefore, while lesional observations in humans have mainly focused on the SC as a cause of RBD, disruptions in other parts of the REM circuitry could also induce RBD, though further clinicopathological and correlative neuroimaging studies are required to confirm this.
Clonazepam
Clonazepam, a long-acting benzodiazepine, has been widely considered as a first-line and highly effective therapy for RBD [84]. The premise for its original use stemmed from its apparent effectiveness in other motor sleep disorders including periodic limb movements of sleep [85]. Several case series and reports have been published in patients with idiopathic/isolated and secondary RBD showing that clonazepam may be effective at suppressing complex and violent dream enactment behaviors [1, 74, 86,87,88]. Presumably, its action relates to enhanced GABAergic and glycinergic inhibition of spinal motor output, consistent with the finding that transgenic mice deficient in these neurotransmitters recapitulate the motor features of RBD [89]. Notably, although clonazepam may decrease the occurrence of sleep-related injury, it does not seem to impact RSWA or elementary vocal or limb jerking body movements associated with RBD in humans, suggesting a “top down” control of dream mentation and enactment behaviors rather than a direct effect on brainstem-mediated REM sleep muscle atonia [88].
The recommended dose for clonazepam in the treatment of RBD is 0.25–3.0 mg taken 30–60 min before bedtime (Table 1). According to case series, injurious motor behaviors may be expected to improve by 67–90% [86, 87, 90]. Tolerance requiring dose escalation does not seem to occur [91]. However, caution must be observed when prescribing clonazepam in elderly populations, where it may increase fall risk and exacerbate cognitive impairment, and in individuals with the especially common comorbidity of sleep-disordered breathing, as it can cause respiratory depression and worsen symptoms [74, 84].
It is important to note that the only randomized placebo-controlled trial in patients with PD and probable RBD failed to show a significant improvement based on a clinical global impression of severity score after 4 weeks of low-dose (0.5 mg) clonazepam [23]. Although there were significant limitations of this study, including the lack of PSG confirmation of RBD, the relatively small sample size, the low dose, and subjective endpoint, this study highlights the need for better assessment tools and large-scale randomized trials to confirm the actual efficacy and optimal dosage regimen of this treatment.
Melatonin
Many experts have considered the alternative treatment of melatonin as the best first-line treatment for RBD, given its tolerability and safety profile and lesser propensity for side effects. Melatonin is an endogenous hormone involved in the regulation of circadian rhythms which is secreted at high levels during the night by the pineal gland, and signals “darkness” to the central suprachiasmastic nucleus and peripheral circadian oscillators [92]. Melatonin was first used as a treatment for RBD in 1995 [93]. Efficacy for melatonin has been reported by several case series [94,95,96,97,98] and one small randomized crossover trial of 8 patients [99]. Dosages of melatonin in these studies ranged from 3 to 12 mg. In the largest comparative study between melatonin and clonazepam including 45 patients, melatonin and clonazepam were reported by patients to be equally effective in reducing RBD severity and frequency, but melatonin was better tolerated with fewer adverse effects and greater injury reduction [98].
In contrast to the studies above, a recent randomized double-blind placebo-controlled trial of slow-release melatonin 4 mg over 8 weeks in 30 patients with PD and RBD found no difference between active or placebo treatment in the primary endpoint of patient-reported RBD incidents recorded by a weekly diary [24]. Possible factors that may account for this negative result included the choice of endpoint, relatively short duration of study, population differences, the low dose of melatonin used, and the type of melatonin formulation. In a previous series, the median effective dose of immediate-release melatonin was 6 mg over a mean duration of 27 months but it also included a heterogenous patient population [98]. The equipoise surrounding melatonin’s efficacy is also mirrored in the limited evidence relating to the use of melatonin agonists such as ramelteon (a melatonin receptor agonist) and agomelatine (a melatonin receptor agonist and antidepressant), which have been associated with a reduction of RBD symptoms in small case series [100, 101] but failed to show a significant benefit in larger series [102]. As with clonazepam, this raises important questions regarding the efficacy of melatonin and melatonergic agents across different RBD groups (isolated, neurodegenerative, antidepressant associated) and the optimal dosage and formulation.
Ultimately, these varied results also reflect our lack of knowledge of the mechanism by which melatonin may interact with the REM atonia pathways discussed above. Indeed, melatonin has been found in some studies to partially restore muscle atonia in REM sleep [95, 99] but the mechanism for this in vitro effect is unknown. Melatonin may act by restoring the circadian timing system and the proper functioning of REM sleep circuitry including atonia control [43]. In relation to PD, disturbances in melatonin secretion and circadian timing have been extensively reported and linked to motor and non-motor symptoms [103, 104]. Work from animal models of PD has suggested melatonin might have a neuroprotective and augmenting effect on dopaminergic neurons [105]. These findings suggest that the degree and manner to which circadian disruption plays a role may vary depending on the underlying cause of RBD, and so melatonin may have variable efficacy via different mechanisms of action across RBD populations. This needs to be considered and explored in future therapeutic studies through more selective recruitment and may be a key to understanding the variable efficacy observed for melatonin, clonazepam, and other symptomatic treatments.
Other Therapies
The quality of evidence for alternative treatments is limited to case series or reports as listed in Tables 1 and 2. Dopaminergic agents, considered by some as a third-line option, have been associated with mixed results. Pramipexole has been shown in case series to be between 62 and 89% effective in certain patients with iRBD [106,107,108] but not in patients with coexisting PD [109]. Rotigotine has also been shown in a recent open-label study to have some efficacy on reported RBD frequency and severity in patients with PD [110]. Acetylcholinesterase inhibitors such as donepezil and rivastigmine may also be considered for use in iRBD, especially in patients with symptoms of cognitive impairment [74, 111]. There are reports of rivastigmine being beneficial in patients with refractory RBD, with either mild cognitive impairment [112] or PD [113]. Thus, it is reasonable to consider acetylcholinesterase inhibitors in patients with RBD and with MCI, dementia with Lewy bodies, or Parkinson’s disease dementia. Recent case series have also suggested some benefit of sodium oxybate, a central nervous system depressant [114,115,116,117] with a placebo-controlled trial currently underway (https://clinicaltrials.gov, NCT04006925). Case reports or small series demonstrating possible efficacy in RBD also exist for temazepam [97], zopiclone [97], carbamazepine [118], gabapentin [119], cannabidiol [120], as well as the herbal preparation Yi-Gan San [121]. Paradoxically, while serotonin-selective reuptake inhibitors are generally considered to exacerbate RBD, paroxetine has been shown in independent case reports to be associated with both improvement and worsening of RBD symptoms [122, 123]. More prospective studies with larger cohorts are required to confirm the efficacy of these alternative therapies, particularly in view of emerging trial evidence casting doubt on the efficacy of the traditional choices of melatonin and clonazepam.
Novel Approaches for Symptomatic Therapy
A common finding across all studies of available pharmacotherapy for RBD is that objective changes in the PSG on RBD measures is rarely seen, even when symptomatic benefit is demonstrated, suggesting a need for innovation in the development of biologically driven approaches. The complex interplay of several regions and neurotransmitters involved in REM atonia control may provide rationale for novel treatment approaches. These include testing of molecules with actions that promote glycinergic neurotransmission, facilitate melanin-concentrating hormone, and modulate orexinergic and noradrenergic pathways. Another important avenue for investigation includes considering combination therapies that act on different pathways simultaneously. This would seem especially pertinent to neurodegenerative diseases such as DLB, where many of the regions implicated in REM sleep regulation can become pathologically affected resulting in a “multiple hit” model of RBD that would require multiple simultaneous therapeutic approaches simultaneously [78]. In either case, ongoing refinements of our scientific understanding of RBD, particularly with respect to specific neuromodulator and neurotransmitter receptor expression in key regions (such as the SC/SLD), will be critical for informing targets for RBD going forward.
Controversy 3: Should RBD Be Treated Equally Across Different Conditions?
RBD is seen across a heterogenous range of neurodegenerative, neurological, primary sleep and psychiatric disorders as well as by antidepressant medications. That RBD can be caused by several distinct pathophysiological processes raises the important question of whether there is, or will ever be, a singular treatment for RBD or whether a more tailored approach will prevail in the management of this condition. Biological distinctions between sites of pathology across different neurodegenerative conditions may lead to RBD via different pathways, with certain conditions potentially being resistant to standard RBD treatment due to multisite involvement and requiring a combination of therapies. Indeed, heterogenous responses to therapy may be suggested simply on the account of the differences in clinical profiles of different RBD populations. One example of this is trauma-associated sleep disorder which has many similar features to RBD, but carries a different demographic profile to patients with iRBD [18]. Although no head-to-head treatment trials exist in trauma-associated sleep disorder, clonazepam has been observed to be less effective in treating RBD compared to established treatments for PTSD such as prazosin or imagery rehearsal therapy [124]. Patients with type 1 narcolepsy who have RBD also carry a different clinical profile [125]. In these patients, the use of sodium oxybate has been shown to be effective in treating RBD and in reducing RSWA, suggesting superiority over clonazepam [126].
Many of the patients in the original iRBD treatment efficacy reports and later clinical trials were older (ages 50–60) men [2, 127], likely due to recruitment bias as men with RBD appear more prone to manifest violent and injurious behavior when presenting to sleep centers [4]. However, in younger populations, RBD occurs more equally in men and women [128,129,130,131]. Early-onset RBD prior to the age of 50–60 years may carry different clinical and therapeutic implications, with a greater frequency of female patients in this younger age group, and greater antidepressant use and comorbid autoimmunity [132]. Practically, symptomatic management tends to focus on managing associated comorbid conditions and medications. In women with iRBD, sleep injuries and disruption may be less common, though a risk of developing a neurodegenerative disorder appears to be equal to that seen in men [22]. These observed differences in clinical characteristics in iRBD suggest biological heterogeneity with a variable and differential risk of phenoconversion to PD, DLB, or MSA, which have overlapping but distinct pathologies. Furthermore, while most older adults with iRBD progress to a synucleinopathy, there are patients who do not convert even after longitudinal follow-up [22, 133], and these patients may represent another biological subgroup within RBD that may respond differently to therapy. Understanding the biological resilience of such a subgroup toward slow progression may hold clues toward future effective neuroprotective interventions.
Ultimately, dedicated studies will be necessary to understand the differential responses to symptomatic and disease-modifying treatments across different phenotypes in patients with iRBD, and in RBD associated with different conditions. Identification of biomarkers that help characterize the heterogeneity of iRBD and its variability in response to symptomatic treatment and in phenoconversion to neurodegenerative disorders is necessary to inform therapeutic trials.
Controversy 4: Antidepressants—Do They Provoke or Unmask RBD?
The degree to which antidepressants provoke RBD de novo, or simply unmask a covert underlying neurodegenerative process, remains a controversial issue. In the former case, the main goal of therapy may be to switch or minimize antidepressants, while the latter may carry additional implications for follow-up and enrollment in neuroprotective trials. Antidepressant classes that have been associated with RBD include tricyclics, serotonin-selective reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors (SNRIs), mirtazapine, and monoamine oxidase inhibitors (MOAIs) [134]. Bupropion, a noradrenaline-dopamine reuptake inhibitor, may be least likely to cause RSWA [135], and anecdotally, may be less likely to cause RBD although there is currently no definitive studies regarding associations between medication-induced RBD and antidepressant subtype. Given the role of noradrenaline and serotonin in the regulation of REM sleep, there is biological plausibility for a direct action of antidepressants on REM atonia [136]. Increased tonic and phasic EMG activity during REM sleep compared to baseline has been demonstrated to occur in patients with depression following administration of sertraline in an open-labeled trial [137]. Increased RSWA in patients taking antidepressants has also been noted in cross-sectional studies [135, 138], with one study finding a higher degree of phasic activity in limb muscles rather than tonic activity in submentalis, which is a hallmark of iRBD in older individuals [135]. Interestingly, Zhang et al. [137] found that the degree of REM atonia in patients taking sertraline correlated with prolonged REM latency. These findings suggest possible diverging mechanisms for RSWA seen with antidepressants compared to that in typical iRBD.
There are case reports of RBD developing following administration of antidepressants [139, 140], yet most patients taking antidepressants never develop RBD. Moreover, many patients with antidepressant-associated RBD also suffer from psychiatric conditions, which also carry an increased risk of developing RBD [141]. As such, quantifying the independent association between antidepressants and RBD is challenging. Studies analyzing the degree of RSWA across RBD populations taking antidepressants have found evidence of a gradient effect, with greater RSWA in typical or traditional RBD (e.g., PD, iRBD) versus non-RBD populations [135, 142], suggesting additional clinical predisposing factors may be required for antidepressants to trigger RBD.
The relationship between antidepressants, depression, and neurodegeneration is still under investigation. Depression itself is a known prodromal symptom of PD and DLB [143] which confounds the proposed link between patients taking antidepressants and RBD caused by an underlying synucleinopathy. Interestingly however, a recent large multicenter cohort study found that depression did not significantly increase the risk of phenoconversion in patients with iRBD [22], implying a possible bidirectional relationship between RBD-related sleep disturbance and depression. Treatment of RBD may improve depressive symptoms [144]. In a prospective cohort study of 100 patients with iRBD including 27 who were using antidepressants, neurodegenerative markers including impaired olfaction, color vision, and mild parkinsonism were equally frequent between patients who received or did not receive antidepressants [145]. However, patients receiving antidepressants were less likely to develop a synucleinopathy at 5 years, possibly explained by a distinction in the underlying biology of antidepressant associated RBD, or alternatively a longer lead time from RBD symptoms to the onset of neurodegeneration if antidepressants are unmasking RBD at an earlier time point.
Large prospective controlled studies are required to assess the true risk of degeneration in those who develop RSWA/RBD while receiving antidepressant medications. In the meantime, careful diagnosis and exclusion of mimics, optimizing environmental precautions, reconsideration of need, type and dosage of antidepressant, use of psychological therapies, and longitudinal neurological and cognitive assessment remain pillars of management in patients requiring treatment for depression.
Controversy 5: How Do We Know if the Treatment Is Working?
Effective methods for monitoring symptom severity are important for deciding on the prescription and titration of therapy in the clinical setting and for defining endpoints for clinical trials. Unfortunately, finding the right measure that balances sensitivity, specificity, and feasibility has been challenging. An initial consensus statement of the International RBD Study Group (RBDSG) proposed the clinical global impression (CGI) efficacy index, which consists of a 4-point scale rating degree of improvement, as a primary endpoint for therapeutic trials [146]. Sleep diaries were also suggested as potential secondary endpoints in studies. However, the variable sensitivity and specificity of self-reporting scales for characterizing RBD severity has led to criticism of recent epidemiological studies and randomized controlled trials that have used such measures [23, 65]. Thus, there has been a shift of opinion away from self-reported measures toward endorsing objective PSG measures as markers of RBD severity [3]. This has been made more feasible by studies showing that certain PSG measures may be robust when comparing night-to-night recordings and the growing accessibility of automated analysis methods. However, the dissociation between lack of change in PSG measures of excessive muscle activity and the self-reported improvement of injurious behaviors that has formed the basis of our most effective therapies needs to be reconciled. Therefore, a combination of endpoints may still be required to determine therapeutic efficacy, with the primary endpoint being dependent on the aim of the therapy (improving injurious behavior versus targeting the underlying disease biology of RSWA). This also highlights an urgent need for adopting more innovative, cost-effective technologies for measuring RBD, especially in the home environment. Such solutions are already being considered in other areas of sleep medicine such as for OSA [147]. Portable and wireless recording sleep systems, smartphone applications, actigraphy wearables, and embedded devices are currently being evaluated to capture and quantify different aspects of sleep and sleep-related behaviors [69], and these methods have only just begun to be applied to patients with RBD specifically [70, 148]. Together with the wide availability of video recording methods, prolonged home monitoring may supplant single night video PSG for the detection of sleep behaviors associated with RBD. With the potential advent of neuroprotective trials, more work is required in this exciting and rapidly evolving space to develop reliable and objective biomarkers for RBD.
Controversy 6: Neuroprotective Treatments in Isolated RBD—Who, When, and How to Treat?
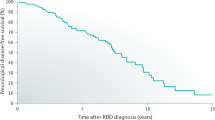
Longitudinal studies have confirmed that approximately 75% of patients with iRBD will develop DLB, PD, or more rarely, MSA [22]. PSG-confirmed RBD carries the highest predictive value (likelihood ratio of 130) compared to any other biomarker for developing a synucleinopathy [149, 150]. With a lack of effective cures for PD, DLB, and MSA, focus has turned onto iRBD as a prodromal stage where disease-modifying therapies could be effective at preventing or delaying the devastating evolution of cognitive, motor, and autonomic nervous system impairments.
Who to Treat?
Much effort has been invested toward identifying biomarkers predictive of phenoconversion risk in patients with RBD [150]. A recent study combining prospective follow-up data of patients with iRBD from 24 centers of the RBDSG calculated the risk of developing dementia and parkinsonism associated with a range of clinical and imaging markers such as hyposmia, motor symptoms, cognitive impairment, and color vision abnormalities [22]. Sample size estimates for conducting definitive neuroprotective trials that would detect a 50% reduced risk of phenoconversion over a 2-year period ranged from 142 to 366 patients per arm depending on the combination of clinical markers used [22]. However, as noted by the authors of this study, there are several limitations to this approach. Importantly, different centers may use different methods to diagnose and measure clinical markers. Additionally, the risk was categorized based on baseline variables, which may not necessarily inform risk if these markers were measured at differing points in time in individual disease course of patients with RBD. Finally, the average follow-up across individual studies was 4.6 years, which is smaller than the median time of phenoconversion (8 years), suggesting that the majority of the iRBD population in most studies had not yet phenoconverted. Despite these limitations, this and other similar studies have taken an important step toward operationalizing a set of criteria for stratifying those at greatest risk of developing neurodegeneration for recruitment into therapeutic trials. However, whether these criteria apply to RBD in the context of antidepressants or psychiatric disorders, and whether such patients should also be included in disease-modifying trials, remains an open question.
When to Treat?
The median time from diagnosis to phenoconversion is around 8 years [22]. Thus, additional factors will need to be taken into consideration when enrolling patients into clinical trials, which, for logistical and economic purposes, rarely exceed 5 years [150]. Studies using patient populations with RBD enriched with multiple risk factors for earlier phenoconversion will be required. However, translating results of such studies to other populations will then be challenging. For instance, only a third of patients with iRBD display motor symptoms [22]. If motor symptoms are used as an inclusion criterion of a clinical trial, the results may not generalize to patients with iRBD but without those symptoms. Another related problem when translating to real-life clinical settings is whether a treatment should be initiated at the onset of symptoms or after sufficient additional features (such as hyposmia, constipation, and motor symptoms) have accumulated to meet the inclusion criteria of a trial or justify the risk of a particular therapy. For example, should patients with isolated RSWA, which could be a form of prodromal RBD in some individuals [3, 34, 37], also be considered an at-risk population that could be candidates for treatment with a neuroprotective therapy? Although there is currently a complete lack of prospective studies analyzing phenoconversion risk in isolated RSWA, this patient group may be reasonably expected to have the least amount of disseminated neurodegeneration in brain. However, this group would also require a considerably longer duration of follow-up to determine whether a candidate neuroprotective therapy was effective for mitigating or slowing neurodegeneration, resulting in reduced feasibility, increased costs and a higher risk of patient exposure to adverse effects of treatment. At any stage, determination of the optimal timing of disease-modifying treatment in RBD will require careful consideration of an individual patient’s risk of conversion and cumulative risk of developing an adverse reaction.
How to Treat?
Establishing methodology for clinical trials in RBD will be challenging, and it also unclear which agents should be tested for disease-modifying properties in patients with iRBD. One approach may be extrapolation from trials conducted in patients with PD.
Several disease-modifying trials for PD have adopted a range of approaches (for a review of current strategies, see the article by Lang and Espay [151]). The greatest interest involves the targeting of α-synuclein, primarily through immunotherapeutic approaches using active and passive immunization with antibodies against specific forms of α-synuclein (including some phase 2 studies) [152]. There is also promise in therapies informed by monogenetic forms of PD including those with mutations in the glucocerebrosidase gene (GBA) and leucine-rich repeat kinase (LRRK2) [153]. Molecular therapies currently targeting the activity of these implicated enzymes will initially be tested on mutation carriers, but there is also evidence for possible broader uses given a signal for efficacy in sporadic forms of PD [154]. So far, most completed trials in PD have been highly discouraging, leading to a call for a global reconceptualization of our pathophysiological understanding of PD and Lewy body diseases [155].
Therapeutic decisions in iRBD involve different benefit-risk profiles than trials in PD. For instance, patients with iRBD are not fully symptomatic with regard to cognitive, motor, or autonomic deficits; have differing trajectories of decline; and may have a decade or more before possibly converting to a synucleinopathy. Thus, neuroprotective agents trialed in iRBD would need to have minimal side effects when taken for a prolonged period and have an excellent established record of safety. Novel compounds may be decades away from being able to satisfy these criteria. Alternatively, “repurposed” medications may be more suitable in iRBD, since the efficacy and safety profile for repurposed drugs has been demonstrated in other conditions, but with theoretical or empirical support (often based on post hoc analyses of epidemiological data) of possible efficacy in PD [156]. Examples in advanced phases of study include calcium channel antagonists [157], urate-lowering therapies [158], and iron-chelating agents [159]. A recently completed randomized double-blind placebo-controlled trial of exenatide in PD demonstrated a small positive effect on the primary endpoint of motor symptom severity in the off-state after 48 weeks [160]. As in PD, analyses of epidemiological data in existing iRBD cohorts may yield insights into which repurposed medications should be prioritized. Another category of intervention that could be assessed in RBD is non-pharmacological approaches such as exercise and nutritional and lifestyle interventions. These have been suggested as influencing the course of neurodegeneration in PD and dementia and may be the most practical to trial in an early, prodromal phase of the disease [161,162,163,164,165]. Recently, higher physical activity has been associated with a reduced risk of probable RBD [164]. Given the accumulating evidence for the neuroprotective effects of exercise on synucleinopathies, it seems prudent to recommend exercise for all patients with RBD while awaiting the development of possible future medical therapies [166]. Further, it will be important for future trials of neuroprotective compounds to consider the potential confounding effects of varying fitness and amounts of exercise in trial participants.
Clearly, iRBD represents a rare opportunity to treat neurodegenerative synucleinopathies before the cardinal features become manifest and extensive neurodegeneration occurs. Repurposing of medications seems to be the most feasible approach, at least initially, before safety data becomes available in novel compounds that have demonstrated efficacy in later stages of the disease. Given the large number of patients required for clinical trials, collaborative efforts should be made to stratify and prioritize candidate therapies for trials in this condition.
Controversy 7: Should We Be Disclosing the Risk of Neurodegeneration to Our Patients?
At the time of diagnosis, most patients with iRBD are otherwise healthy and the disclosure of neurodegenerative risk raises several ethical issues, particularly considering the variable trajectories of individuals, lack of definitive treatment, and the possibility that some patients may never convert to a synucleinopathy. There are no systematic studies yet to guide prognosis disclosure to patients with RBD, and especially, patients with isolated RSWA. The ethical discourse surrounding disclosure of risk is that it should be individualized, respecting the patient’s personality, education, cultural heritage, social characteristics, and religious beliefs, whilst being guided by the principles of autonomy, beneficence, non-maleficence, and justice (for a recent discussion, see the article by Arnaldi et al. [167]). Once these aspects have been considered, for most cases of iRBD, disclosure of risk of neurodegeneration may be appropriate when patients express a desire to learn about this risk, while acknowledging the significant uncertainty that applies at an individual level. Counseling patients about phenoconversion risk provides them with an opportunity to be aware of the emergence of potential symptoms of synucleinopathy and seek timely symptomatic treatment. Disclosure of risk may also inform future life planning and enables patients to consider participation in future neuroprotective studies. Furthermore, some evidence already exists for the benefits of exercise and nutrition/dietary changes in lowering the risk of neurodegeneration [163, 165]. The impending risk of neurodegeneration may encourage some patients to be motivated to undertake otherwise favorable and healthy lifestyle changes. Finally, most patients will likely encounter the information about their risk from other sources on the Internet, and therefore, timely disclosure affords physicians an opportunity to share correct information and to direct the patient to reliable resources.
Concluding Remarks and Future Directions
The present review summarizes and highlights current concepts underlying therapy for RBD and the important challenges to be addressed that will pave the way for the much anticipated and greatly needed therapeutic trials. Some of the remaining big questions are summarized in Table 3. Randomized controlled trials are needed for existing symptomatic therapies, while also taking advantage of recent insights gained into the biological underpinnings of RBD to develop novel treatments. Therapeutic trials need to also consider the possible biological heterogeneity of RBD and to define treatment effects in specific patient subgroups. The risk of neurodegeneration in patients with medication-induced RBD, especially those receiving antidepressants and with concomitant mood disorders, will need to be resolved by prospective cohort studies. Further work is also needed to develop more objective biomarkers for diagnostic and disease-monitoring purposes with a focus on novel technological approaches. Finally, the optimal design of disease-modifying trials in RBD is an ongoing discussion with emerging and future work set to inform critical questions regarding recruitment, timing of therapy, and the type of neuroprotective intervention.
References
Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293-308.
Fernández-Arcos A, Iranzo A, Serradell M, Gaig C, Santamaria J. The Clinical Phenotype of Idiopathic Rapid Eye Movement Sleep Behavior Disorder at Presentation: A Study in 203 Consecutive Patients. Sleep. 2016;39(1):121-32.
Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol. 2018;14(1):40-55.
Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016;15(4):405-19.
Gagnon JF, Bédard MA, Fantini ML, Petit D, Panisset M, Rompré S, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59(4):585-9.
Scaglione C, Vignatelli L, Plazzi G, Marchese R, Negrotti A, Rizzo G, et al. REM sleep behaviour disorder in Parkinson's disease: a questionnaire-based study. Neurol Sci. 2005;25(6):316-21.
Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14(8):754-62.
Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-30.
Palma JA, Fernandez-Cordon C, Coon EA, Low PA, Miglis MG, Jaradeh S, et al. Prevalence of REM sleep behavior disorder in multiple system atrophy: a multicenter study and meta-analysis. Clin Auton Res. 2015;25(1):69-75.
Nightingale S, Orgill JC, Ebrahim IO, de Lacy SF, Agrawal S, Williams AJ. The association between narcolepsy and REM behavior disorder (RBD). Sleep Med. 2005;6(3):253-8.
Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133(Pt 2):568-79.
Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575-86.
Iranzo A, Graus F, Clover L, Morera J, Bruna J, Vilar C, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59(1):178-81.
Cornelius JR, Pittock SJ, McKeon A, Lennon VA, Aston PA, Josephs KA, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68(6):733-8.
Devine MF, Feemster JC, Lieske EA, McCarter SJ, Sandness DJ, Steele T, et al. 0003 LGI1 and CASPR2 Autoimmunity: Sleep Symptoms, Polysomnography, and Quantitative REM Sleep without Atonia. Sleep. 2020;43(Supplement_1):A1-A2.
Iranzo A, Aparicio J. A lesson from anatomy: focal brain lesions causing REM sleep behavior disorder. Sleep Med. 2009;10(1):9-12.
McCarter SJ, Tippmann-Peikert M, Sandness DJ, Flanagan EP, Kantarci K, Boeve BF, et al. Neuroimaging-evident lesional pathology associated with REM sleep behavior disorder. Sleep Med. 2015;16(12):1502-10.
Rachakonda TD, Balba NM, Lim MM. Trauma-Associated Sleep Disturbances: a Distinct Sleep Disorder? Current Sleep Medicine Reports. 2018;4(2):143-8.
McCarter SJ, Feemster JC, Tabatabai GM, Sandness DJ, Timm PC, McCarter AR, et al. Submentalis Rapid Eye Movement Sleep Muscle Activity: A Potential Biomarker for Synucleinopathy. Ann Neurol. 2019;86(6):969-74.
McCarter SJ, Tabatabai GM, Jong HY, Sandness DJ, Timm PC, Johnson KL, et al. REM sleep atonia loss distinguishes synucleinopathy in older adults with cognitive impairment. Neurology. 2020;94(1):e15-e29.
Iranzo A, Fernández-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9(2):e89741.
Postuma RB, Iranzo A, Hu M, Hogl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744-59.
Shin C, Park H, Lee WW, Kim HJ, Kim HJ, Jeon B. Clonazepam for probable REM sleep behavior disorder in Parkinson's disease: A randomized placebo-controlled trial. J Neurol Sci. 2019;401:81-6.
Gilat M, Coeytaux Jackson A, Marshall NS, Hammond D, Mullins AE, Hall JM, et al. Melatonin for rapid eye movement sleep behavior disorder in Parkinson's disease: A randomised controlled trial. Mov Disord. 2020;35(2):344-9.
Medicine AAoS. International classification of sleep disorders - third edition.: Darien: American Academy of Sleep Medicine; 2014.; 2014.
Berry RB, Brooks R, Gamaldo CE, Harding SM, M LR, Quan SF, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. ed: Darien, IL: American Academy of Sleep Medicine; 2017. 2020.
Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42(7):1371-4.
McCarter SJ, St Louis EK, Duwell EJ, Timm PC, Sandness DJ, Boeve BF, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep. 2014;37(10):1649-62.
Bliwise DL, Rye DB. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep. 2008;31(6):853-7.
Frauscher B, Iranzo A, Högl B, Casanova-Molla J, Salamero M, Gschliesser V, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31(5):724-31.
Frauscher B, Iranzo A, Gaig C, Gschliesser V, Guaita M, Raffelseder V, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835-47.
Iranzo A, Frauscher B, Santos H, Gschliesser V, Ratti L, Falkenstetter T, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12(3):284-8.
McCarter SJ, St Louis EK, Sandness DJ, Duwell EJ, Timm PC, Boeve BF, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med. 2017;33:23-9.
Feemster JC, Jung Y, Timm PC, Westerland SM, Gossard TR, Teigen LN, et al. Normative and isolated rapid eye movement sleep without atonia in adults without REM sleep behavior disorder. Sleep. 2019;42(10).
Ferri R, Fulda S, Cosentino FI, Pizza F, Plazzi G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson's disease with or without REM sleep behavior disorder. Sleep Med. 2012;13(6):707-13.
Ferri R, Manconi M, Plazzi G, Bruni O, Vandi S, Montagna P, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. Journal of Sleep Research. 2008;17(1):89-100.
Stefani A, Gabelia D, Högl B, Mitterling T, Mahlknecht P, Stockner H, et al. Long-Term Follow-up Investigation of Isolated Rapid Eye Movement Sleep Without Atonia Without Rapid Eye Movement Sleep Behavior Disorder: A Pilot Study. J Clin Sleep Med. 2015;11(11):1273-9.
McCarter SJ, Sandness DJ, McCarter AR, Feemster JC, Teigen LN, Timm PC, et al. REM sleep muscle activity in idiopathic REM sleep behavior disorder predicts phenoconversion. Neurology. 2019;93(12):e1171-e9.
Nepozitek J, Dostalova S, Dusek P, Kemlink D, Prihodova I, Ibarburu Lorenzo YLV, et al. Simultaneous tonic and phasic REM sleep without atonia best predicts early phenoconversion to neurodegenerative disease in idiopathic REM sleep behavior disorder. Sleep. 2019.
Fernández-Arcos A, Iranzo A, Serradell M, Gaig C, Guaita M, Salamero M, et al. Diagnostic Value of Isolated Mentalis Versus Mentalis Plus Upper Limb Electromyography in Idiopathic REM Sleep Behavior Disorder Patients Eventually Developing a Neurodegenerative Syndrome. Sleep. 2017;40(4).
Frauscher B, Gabelia D, Biermayr M, Stefani A, Hackner H, Mitterling T, et al. Validation of an integrated software for the detection of rapid eye movement sleep behavior disorder. Sleep. 2014;37(10):1663-71.
Cesari M, Christensen JAE, Sorensen HBD, Jennum P, Mollenhauer B, Muntean ML, et al. External validation of a data-driven algorithm for muscular activity identification during sleep. Journal of Sleep Research. 2019;28(6):e12868.
Schenck CH, Hogl B, Videnovic A. Rapid-Eye-Movement Sleep Behavior Disorder. 1 ed: Springer International Publishing; 2019.
Cygan F, Oudiette D, Leclair-Visonneau L, Leu-Semenescu S, Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med. 2010;6(6):551-5.
Ferri R, Marelli S, Cosentino FI, Rundo F, Ferini-Strambi L, Zucconi M. Night-to-night variability of automatic quantitative parameters of the chin EMG amplitude (Atonia Index) in REM sleep behavior disorder. J Clin Sleep Med. 2013;9(3):253-8.
Zhang J, Lam SP, Ho CK, Li AM, Tsoh J, Mok V, et al. Diagnosis of REM sleep behavior disorder by video-polysomnographic study: is one night enough? Sleep. 2008;31(8):1179-85.
Ferri R, Franceschini C, Zucconi M, Vandi S, Poli F, Bruni O, et al. Searching for a marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep. 2008;31(10):1409-17.
Ferri R, Rundo F, Manconi M, Plazzi G, Bruni O, Oldani A, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med. 2010;11(9):947-9.
Frandsen R, Nikolic M, Zoetmulder M, Kempfner L, Jennum P. Analysis of automated quantification of motor activity in REM sleep behaviour disorder. Journal of Sleep Research. 2015;24(5):583-90.
Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22(16):2386-93.
Li SX, Wing YK, Lam SP, Zhang J, Yu MW, Ho CK, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med. 2010;11(1):43-8.
Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin SC, Bieniek K, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12(5):445-53.
Frauscher B, Ehrmann L, Zamarian L, Auer F, Mitterling T, Gabelia D, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012;27(13):1673-8.
Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913-6.
Schenck CH, Hurwitz TD, Mahowald MW. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. Journal of Sleep Research. 1993;2(4):224-31.
Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147-52.
Frauscher B, Gabelia D, Mitterling T, Biermayr M, Bregler D, Ehrmann L, et al. Motor events during healthy sleep: a quantitative polysomnographic study. Sleep. 2014;37(4):763-73, 73a-73b.
Iranzo A, Santamaría J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28(2):203-6.
Gaig C, Iranzo A, Pujol M, Perez H, Santamaria J. Periodic Limb Movements During Sleep Mimicking REM Sleep Behavior Disorder: A New Form of Periodic Limb Movement Disorder. Sleep. 2017;40(3).
Boeve BF, Molano JR, Ferman TJ, Lin SC, Bieniek K, Tippmann-Peikert M, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. 2013;9(5):475-80.
Bolitho SJ, Naismith SL, Terpening Z, Grunstein RR, Melehan K, Yee BJ, et al. Investigating rapid eye movement sleep without atonia in Parkinson's disease using the rapid eye movement sleep behavior disorder screening questionnaire. Mov Disord. 2014;29(6):736-42.
Stiasny-Kolster K, Sixel-Döring F, Trenkwalder C, Heinzel-Gutenbrunner M, Seppi K, Poewe W, et al. Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson's disease. Sleep Med. 2015;16(1):186-9.
Nomura T, Tanaka K, Tajiri Y, Kishi M, Nakashima K. Screening tools for clinical characteristics of probable REM sleep behavior disorder in patients with Parkinson's disease. eNeurologicalSci. 2016;4:22-4.
Stefani A, Mahlknecht P, Seppi K, Nocker M, Mair KJ, Hotter A, et al. Consistency of "Probable RBD" Diagnosis with the RBD Screening Questionnaire: A Follow-up Study. Movement Disorders Clinical Practice. 2017;4(3):403-5.
Zhang J, Li SX, Lam SP, Wing YK. Epidemiology of REM sleep behavior disorder: both study design and measurement tool count. Sleep Med. 2017;40:122-3.
Wang Y, Wang ZW, Yang YC, Wu HJ, Zhao HY, Zhao ZX. Validation of the rapid eye movement sleep behavior disorder screening questionnaire in China. J Clin Neurosci. 2015;22(9):1420-4.
Nomura T, Inoue Y, Kagimura T, Uemura Y, Nakashima K. Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson's disease patients. Sleep Med. 2011;12(7):711-3.
Matar E, Ehgoetz Martens KA, Halliday GM, Lewis SJG. Clinical features of Lewy body dementia: insights into diagnosis and pathophysiology. J Neurol. 2019.
Ko PR, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer Sleep Technologies: A Review of the Landscape. J Clin Sleep Med. 2015;11(12):1455-61.
Stefani A, Heidbreder A, Brandauer E, Guaita M, Neier LM, Mitterling T, et al. Screening for idiopathic REM sleep behavior disorder: usefulness of actigraphy. Sleep. 2018;41(6).
Howell MJ, Arneson PA, Schenck CH. A novel therapy for REM sleep behavior disorder (RBD). J Clin Sleep Med. 2011;7(6):639-44a.
Schenck CH, Lee SA, Bornemann MA, Mahowald MW. Potentially lethal behaviors associated with rapid eye movement sleep behavior disorder: review of the literature and forensic implications. Journal of Forensic Sciences. 2009;54(6):1475-84.
McCarter SJ, St Louis EK, Boswell CL, Dueffert LG, Slocumb N, Boeve BF, et al. Factors associated with injury in REM sleep behavior disorder. Sleep Med. 2014;15(11):1332-8.
Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. 2010;6(1):85-95.
Scammell TE, Arrigoni E, Lipton JO. Neural Circuitry of Wakefulness and Sleep. Neuron. 2017;93(4):747-65.
Limousin N, Dehais C, Gout O, Héran F, Oudiette D, Arnulf I. A brainstem inflammatory lesion causing REM sleep behavior disorder and sleepwalking (parasomnia overlap disorder). Sleep Med. 2009;10(9):1059-62.
Xi Z, Luning W. REM sleep behavior disorder in a patient with pontine stroke. Sleep Med. 2009;10(1):143-6.
Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(Pt 11):2770-88.
Luppi PH, Clement O, Sapin E, Peyron C, Gervasoni D, Leger L, et al. Brainstem mechanisms of paradoxical (REM) sleep generation. Pflugers Archiv : European journal of physiology. 2012;463(1):43-52.
Sakai K, Crochet S, Onoe H. Pontine structures and mechanisms involved in the generation of paradoxical (REM) sleep. Archives italiennes de biologie. 2001;139(1-2):93-107.
Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8(12):4790-6.
Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589-94.
Lee ML, Swanson BE, de la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Current Biology : CB. 2009;19(10):848-52.
Jung Y, St Louis EK. Treatment of REM Sleep Behavior Disorder. Curr Treat Options Neurol. 2016;18(11):50.
Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder. A treatable parasomnia affecting older adults. JAMA. 1987;257(13):1786-9.
Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25(2):120-38.
Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123 (Pt 2):331-9.
Ferri R, Marelli S, Ferini-Strambi L, Oldani A, Colli F, Schenck CH, et al. An observational clinical and video-polysomnographic study of the effects of clonazepam in REM sleep behavior disorder. Sleep Med. 2013;14(1):24-9.
Brooks PL, Peever JH. Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice. J Neurosci. 2011;31(19):7111-21.
Li SX, Lam SP, Zhang J, Yu MW, Chan JW, Liu Y, et al. A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med. 2016;21:114-20.
Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. The American Journal of Medicine. 1996;100(3):333-7.
Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pract Neurol. 2008;4(8):436-47.
Kunz D, Bes F. Melatonin effects in a patient with severe REM sleep behavior disorder: case report and theoretical considerations. Neuropsychobiology. 1997;36(4):211-4.
Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14(3):507-11.
Takeuchi N, Uchimura N, Hashizume Y, Mukai M, Etoh Y, Yamamoto K, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55(3):267-9.
Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-4.
Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med. 2009;5(3):235-9.
McCarter SJ, Boswell CL, St Louis EK, Dueffert LG, Slocumb N, Boeve BF, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14(3):237-42.
Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. Journal of Sleep Research. 2010;19(4):591-6.
Nomura T, Kawase S, Watanabe Y, Nakashima K. Use of ramelteon for the treatment of secondary REM sleep behavior disorder. Intern Med. 2013;52(18):2123-6.
Bonakis A, Economou NT, Papageorgiou SG, Vagiakis E, Nanas S, Paparrigopoulos T. Agomelatine may improve REM sleep behavior disorder symptoms. J Clin Psychopharmacol. 2012;32(5):732-4.
Esaki Y, Kitajima T, Koike S, Fujishiro H, Iwata Y, Tsuchiya A, et al. An Open-Labeled Trial of Ramelteon in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. J Clin Sleep Med. 2016;12(5):689-93.
Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014;15(3):342-7.
Videnovic A, Lazar AS, Barker RA, Overeem S. 'The clocks that time us'--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683-93.
Mack JM, Schamne MG, Sampaio TB, Pértile RA, Fernandes PA, Markus RP, et al. Melatoninergic System in Parkinson's Disease: From Neuroprotection to the Management of Motor and Nonmotor Symptoms. Oxidative Medicine and Cellular Longevity. 2016;2016:3472032.
Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61(10):1418-20.
Schmidt MH, Koshal VB, Schmidt HS. Use of pramipexole in REM sleep behavior disorder: results from a case series. Sleep Med. 2006;7(5):418-23.
Sasai T, Inoue Y, Matsuura M. Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. The Tohoku Journal of Experimental Medicine. 2012;226(3):177-81.
Kumru H, Iranzo A, Carrasco E, Valldeoriola F, Marti MJ, Santamaria J, et al. Lack of effects of pramipexole on REM sleep behavior disorder in Parkinson disease. Sleep. 2008;31(10):1418-21.
Wang Y, Yang Y, Wu H, Lan D, Chen Y, Zhao Z. Effects of Rotigotine on REM Sleep Behavior Disorder in Parkinson Disease. J Clin Sleep Med. 2016;12(10):1403-9.
Ringman JM, Simmons JH. Treatment of REM sleep behavior disorder with donepezil: a report of three cases. Neurology. 2000;55(6):870-1.
Brunetti V, Losurdo A, Testani E, Lapenta L, Mariotti P, Marra C, et al. Rivastigmine for refractory REM behavior disorder in mild cognitive impairment. Curr Alzheimer Res. 2014;11(3):267-73.
Di Giacopo R, Fasano A, Quaranta D, Della Marca G, Bove F, Bentivoglio AR. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson's disease. Mov Disord. 2012;27(4):559-61.
Shneerson JM. Successful treatment of REM sleep behavior disorder with sodium oxybate. Clin Neuropharmacol. 2009;32(3):158-9.
Liebenthal J, Valerio J, Ruoff C, Mahowald M. A Case of Rapid Eye Movement Sleep Behavior Disorder in Parkinson Disease Treated With Sodium Oxybate. JAMA Neurol. 2016;73(1):126-7.
Moghadam KK, Pizza F, Primavera A, Ferri R, Plazzi G. Sodium oxybate for idiopathic REM sleep behavior disorder: a report on two patients. Sleep Med. 2017;32:16-21.
Mayer G. Efficacy of sodium oxybate on REM sleep behavior disorder in a patient with narcolepsy type 1. Neurology. 2016;87(24):2594-5.
Bamford CR. Carbamazepine in REM sleep behavior disorder. Sleep. 1993;16(1):33-4.
Abenza Abildúa MJ, Miralles Martinez A, Arpa Gutiérrez FJ, Lores Gutiérrez V, Algarra Lucas C, Jimeno Montero C, et al. Conditions associated with REM sleep behaviour disorder: Description of a hospital series. Neurologia. 2019;34(3):159-64.
Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. Journal of Clinical Pharmacy and Therapeutics. 2014;39(5):564-6.
Shinno H, Kamei M, Nakamura Y, Inami Y, Horiguchi J. Successful treatment with Yi-Gan San for rapid eye movement sleep behavior disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1749-51.
Takahashi T, Mitsuya H, Murata T, Murayama J, Wada Y. Opposite effects of SSRIs and tandospirone in the treatment of REM sleep behavior disorder. Sleep Med. 2008;9(3):317-9.
Parish JM. Violent dreaming and antidepressant drugs: or how paroxetine made me dream that I was fighting Saddam Hussein. J Clin Sleep Med. 2007;3(5):529-31.
Mysliwiec V, O'Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-8.
Dauvilliers Y, Jennum P, Plazzi G. Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy. Sleep Med. 2013;14(8):775-81.
Mayer G, Rodenbeck A, Kesper K. Sodium oxybate treatment in narcolepsy and its effect on muscle tone. Sleep Med. 2017;35:1-6.
Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296-300.
Bodkin CL, Schenck CH. Rapid eye movement sleep behavior disorder in women: relevance to general and specialty medical practice. Journal of Women's Health (2002). 2009;18(12):1955-63.
Wong JC, Li J, Pavlova M, Chen S, Wu A, Wu S, et al. Risk factors for probable REM sleep behavior disorder: A community-based study. Neurology. 2016;86(14):1306-12.
Frauscher B, Gschliesser V, Brandauer E, Marti I, Furtner MT, Ulmer H, et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11(2):167-71.
Haba-Rubio J, Frauscher B, Marques-Vidal P, Toriel J, Tobback N, Andries D, et al. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep. 2018;41(2).
Ju YE, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12(3):278-83.
Iranzo A, Stefani A, Serradell M, Martí MJ, Lomeña F, Mahlknecht P, et al. Characterization of patients with longstanding idiopathic REM sleep behavior disorder. Neurology. 2017;89(3):242-8.
Hoque R, Chesson AL, Jr. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6(1):79-83.
McCarter SJ, St Louis EK, Sandness DJ, Arndt K, Erickson M, Tabatabai G, et al. Antidepressants Increase REM Sleep Muscle Tone in Patients with and without REM Sleep Behavior Disorder. Sleep. 2015;38(6):907-17.
Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15-54.
Zhang B, Hao Y, Jia F, Tang Y, Li X, Liu W, et al. Sertraline and rapid eye movement sleep without atonia: an 8-week, open-label study of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:85-92.
Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;27(2):317-21.
Schenck CH, Mahowald MW, Kim SW, O'Connor KA, Hurwitz TD. Prominent eye movements during NREM sleep and REM sleep behavior disorder associated with fluoxetine treatment of depression and obsessive-compulsive disorder. Sleep. 1992;15(3):226-35.
Attarian HP, Schenck CH, Mahowald MW. Presumed REM sleep behavior disorder arising from cataplexy and wakeful dreaming. Sleep Med. 2000;1(2):131-3.
Lam SP, Zhang J, Tsoh J, Li SX, Ho CK, Mok V, et al. REM sleep behavior disorder in psychiatric populations. J Clin Psychiatry. 2010;71(8):1101-3.
Lam SP, Li SX, Chan JW, Mok V, Tsoh J, Chan A, et al. Does rapid eye movement sleep behavior disorder exist in psychiatric populations? A clinical and polysomnographic case-control study. Sleep Med. 2013;14(8):788-94.
Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord. 2012;27(5):617-26.
Chiu NKH, Ehgoetz Martens KA, Mok VCT, Lewis SJG, Matar E. Prevalence and predictors of mood disturbances in idiopathic REM sleep behaviour disorder. Journal of Sleep Research. 2020:e13040.
Postuma RB, Gagnon JF, Tuineaig M, Bertrand JA, Latreille V, Desjardins C, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 2013;36(11):1579-85.
Schenck CH, Montplaisir JY, Frauscher B, Hogl B, Gagnon JF, Postuma R, et al. Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy--a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med. 2013;14(8):795-806.
Penzel T, Schöbel C, Fietze I. New technology to assess sleep apnea: wearables, smartphones, and accessories. F1000Res. 2018;7:413.
Arora S, Baig F, Lo C, Barber TR, Lawton MA, Zhan A, et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology. 2018;91(16):e1528-e38.
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30(12):1600-11.
Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015;84(11):1104-13.
Lang AE, Espay AJ. Disease Modification in Parkinson's Disease: Current Approaches, Challenges, and Future Considerations. Mov Disord. 2018;33(5):660-77.
Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting Alpha-Synuclein as a Therapy for Parkinson's Disease. Frontiers in Molecular Neuroscience. 2019;12:299.
Sardi SP, Simuni T. New Era in disease modification in Parkinson's disease: Review of genetically targeted therapeutics. Parkinsonism Relat Disord. 2019;59:32-8.
Schneider SA, Alcalay RN. Precision medicine in Parkinson’s disease: emerging treatments for genetic Parkinson’s disease. Journal of Neurology. 2020;267(3):860-9.
Espay AJ, Kalia LV, Gan-Or Z, Williams-Gray CH, Bedard PL, Rowe SM, et al. Disease modification and biomarker development in Parkinson disease. Neurology. 2020;94(11):481.
Athauda D, Foltynie T. Drug Repurposing in Parkinson's Disease. CNS Drugs. 2018;32(8):747-61.
Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Annals of Neurology. 2010;67(5):600-6.
Schwarzschild MA, Macklin EA, Bakshi R, Battacharyya S, Logan R, Espay AJ, et al. Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial. Neurology. 2019;93(14):e1328.
Moreau C, Duce JA, Rascol O, Devedjian JC, Berg D, Dexter D, et al. Iron as a therapeutic target for Parkinson's disease. Mov Disord. 2018;33(4):568-74.
Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664-75.
Seidl SE, Santiago JA, Bilyk H, Potashkin JA. The emerging role of nutrition in Parkinson's disease. Front Aging Neurosci. 2014;6:36.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: An umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1-9.
Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas NJMD. The association between Mediterranean diet adherence and Parkinson's disease. Mov Disord. 2012;27(6):771-4.
Hughes KC, Gao X, Molsberry S, Valeri L, Schwarzschild MA, Ascherio A. Physical activity and prodromal features of Parkinson disease. Neurology. 2019;93(23):e2157-e69.
Yang F, Trolle Lagerros Y, Bellocco R, Adami H-O, Fang F, Pedersen NL, et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain. 2014;138(2):269-75.
McCarter SJ, Boeve BF, Graff-Radford NR, Silber MH, St Louis EK. Neuroprotection in idiopathic REM sleep behavior disorder: a role for exercise? Sleep. 2019;42(6).
Arnaldi D, Antelmi E, St Louis EK, Postuma RB, Arnulf I. Idiopathic REM sleep behavior disorder and neurodegenerative risk: To tell or not to tell to the patient? How to minimize the risk? Sleep Medicine Reviews. 2017;36:82-95.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
E.M. is a recipient of scholarships from the National Health and Medical Research Council (NHMRC), the Australian and New Zealand Association of Neurologists (Gwen James Fellowship). EKS was supported by NIH/NCRR/NCATS CCaTS Grant Number UL1 TR002377 and R34AG056639. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. S.J.G.L. is supported by a NHMRC-Australian Research Council Dementia Research Development fellowship (#1110414).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
EM reports no conflicts of interest or relevant disclosures. SJM reports no conflicts of interest or relevant disclosures. EKSL reports no conflicts of interest or relevant disclosures. SJGL reports no conflicts of interest or relevant disclosures.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1224 kb)
Rights and permissions
About this article
Cite this article
Matar, E., McCarter, S.J., St Louis, E.K. et al. Current Concepts and Controversies in the Management of REM Sleep Behavior Disorder. Neurotherapeutics 18, 107–123 (2021). https://doi.org/10.1007/s13311-020-00983-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-020-00983-7