Abstract
Lower sepsis mortality rates imply that more patients are discharged from the hospital, but sepsis survivors often experience sequelae, such as functional disability, cognitive impairment, and psychiatric morbidity. Nevertheless, the mechanisms underlying these long-term disabilities are not fully understood. Considering the extensive use of animal models in the study of the pathogenesis of neuropsychiatric disorders, it seems adopting this approach to improve our knowledge of postseptic psychiatric symptoms is a logical approach. With the purpose of gathering and summarizing the main findings of studies using animal models of sepsis-induced psychiatric symptoms, we performed a systematic review of the literature on this topic. Thus, 140 references were reviewed, and most of the published studies suggested a time-dependent recovery from behavior alterations, despite the fact that some molecular alterations persist in the brain. This review reveals that animal models can be used to understand the mechanisms that underlie anxiety and depression in animals recovering from sepsis.
Similar content being viewed by others
Introduction
Although the number of deaths associated with sepsis has decreased in recent decades [1,2,3], sepsis is still a major health problem worldwide [4]. Lower mortality rates imply that more patients are discharged from the hospital, but sepsis survivors often experience sequelae, such as functional disability, cognitive impairment, and psychiatric morbidity [5, 6]. Few interventions have been designed to improve quality of life among survivors of sepsis [7,8,9,10,11], often with poor results. Nevertheless, the mechanisms that result in these long-term disabilities are not fully understood [12,13,14,15]. In this context, animal models are often important to use in understanding the mechanisms of these long-term disabilities and to develop new therapeutic strategies for these patients.
Psychiatric syndromes are often observed in sepsis survivors. Patients discharged from an intensive care unit (ICU) report a high prevalence of anxiety, depression, and posttraumatic stress disorder (PTSD) symptoms [5]. Recent meta-analyses indicate that one-third of ICU survivors develop anxiety and depressive symptoms, and one-fifth experience clinical PTSD symptoms [16,17,18]. All of these psychiatric disorders can persist for 1 year or more and are more prevalent in patients who had preexisting psychopathologies, presented psychological disorders at ICU admission, and had memories of delusional experiences during their ICU stay [16,17,18]. Interestingly, most ICU-related factors—such as severity of illness, diagnosis, and length of stay—have a slight impact on long-term psychiatric outcomes [16,17,18]. However, some observational studies have pointed out sepsis as an independent risk factor for stress disorders after critical illness [19,20,21].
It seems clear that sepsis elicits long-term psychiatric symptoms/syndromes that worsen a patient’s quality of life, and it is still not clear whether their mechanisms are the same as those of the depressive and anxiety disorders of psychiatric patients. The effectiveness of classic treatments in the sepsis survivor population is also still in question. For example, a retrospective study showed that the de novo initiation of antidepressant medications in patients who were in treatment before ICU admission did not substantially decrease the prevalence of post-ICU depression [22].
Considering the extensive use of animal models in the study of the pathogenesis of neuropsychiatric disorders [23,24,25], it seems relevant to adopt this approach to improve our knowledge on postseptic psychiatric symptoms. This could also lead to the identification of effective therapies to preemptively prevent or treat candidates for post-ICU psychiatric symptoms. With the purpose of gathering and summarizing the main findings of studies using animal models of sepsis-induced psychiatric symptoms, we performed a systematic review and a critical appraisal of the literature on this topic.
Methods
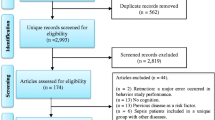
We searched the PubMed and Medline databases for papers published between 1975 and December 2018 using combinations of words or terms that included “sepsis[MeSH Terms] OR sepsis-associated encephalopathy[MeSH Terms] OR systemic inflammatory response syndrome [MeSH Terms] OR endotoxemia[MeSH Terms] OR lipopolysaccharides (LPS) [MeSH Terms] OR cecal ligation[MeSH Terms]” AND “mental disorders[MeSH Terms] OR anxiety[MeSH Terms] OR anxiety disorders[MeSH Terms] OR mental health[MeSH Terms] OR depression[MeSH Terms] OR depressive disorder[MeSH Terms] OR affective disorder[MeSH Terms] OR stress disorder[MeSH Terms] OR mood disorder[MeSH Terms] OR attention[MeSH Terms]” limited in species to “other animals.” Two independent investigators identified relevant articles by reading their titles and abstracts and included any article that used as an outcome a task that measures affective domains (for example, anxiety, depression, PTSD). Tasks that overlapped cognitive and affective domains were not included. If there was no agreement between the 2 investigators, a third researcher gave the final decision. Abstracts and articles that were not written in English were excluded. The selected publications were then read, and the pertinent publications were identified. Among these criteria, 6279 publications were identified, 284 of which were selected based on their titles and 140 of which were chosen based on the manuscripts. From these 140 articles, 7 used a low-LPS dose to investigate acute (few minutes/hour after LPS injection) sickness behavior, and therefore, these were not included in the analyses.
Results
Different animal models usually look at different behavioral domains, such as locomotion/exploration, defensive responses, anhedonia, and attempt to escape. In this sense, the use of different models is usually necessary to fully characterize a behavioral phenotype or the effect of a specific intervention. In addition, acknowledging the influence of environmental and animal-related aspects—such as species, age, sex, diet, housing situation, and stress levels—is of great importance when working with animal models. It is important to note that most models of rodent behavior have been optimized for the rat. Yet, due to the not-so-recent expansion of the mouse model, mainly due to its success in the genetic engineering field, these models were adapted to mice testing.
Anxiety
Anxiety and fear produce similar behavioral responses, including increased vigilance, freezing and/or hypoactivity, elevated heart rate, and suppressed food consumption [26]. These traits are crucial to survival and are therefore highly conserved throughout evolution, facilitating the recognition and extrapolation of anxiety-related behavior from rodents to humans. Overall, the literature suggests that the amygdala mediates fear-like behaviors to short, discrete, and proximal aversive cues, whereas the bed nucleus of the stria terminalis (BNST) mediates anxiety-like or worry-like behaviors [27], but area specificity could also be influenced by the specificity of each anxiety task. Over a dozen different tasks are available for studying anxiety and drug discovery for anxiety treatment [28], in addition to several adaptations of these tasks. Among these, 3 anxiety-related defense behavior assays that specifically aim to measure rodent anxiety have been widely adopted, which are also referred to as “approach-avoidance conflict tests”: the elevated plus maze (EPM), the light–dark box (LDB), and the open field (OF) [28]. All 3 tests measure unconditioned responses based on the innate conflict between the animals’ natural drive to explore novelty and their aversion toward elevated, bright, and open zones, respectively [29]. A recent meta-analysis investigated the effects of diazepam on these 3 anxiety paradigms and revealed a large effect of this drug on these tests [25], most consistently observed in EPM and LDB [25]. It is important to note that there is strong EPM-LDB and OF-LDB assay concordance, but EPM and OF do not reproduce each other’s evaluation of anxiety [25]. Other less frequently used models of unconditioned responses linked to anxiety include the predator odor aversion, measures of ultrasound vocalization (after maternal separation, for example), novelty suppressed feeding, the hole-board test, and the marble burying test. Furthermore, some models of conditioned responses have also been developed. They usually involve the pairing of a previously neutral stimulus with an aversive one, and the resulting avoidance/defense behavior is the output associated with anxiety. These tests are not the first choice for the study of anxiety due to confounding effects of motivational and perceptual states arising from interference with learning/memory, hunger/thirst, or nociceptive mechanisms intrinsic to these models [30].
It has been empirically suggested that inconsistencies between anxiety tests may result from the influence of environmental factors, including animal suppliers, handling experimenters, apparatus structure and color, illumination and light/dark cycle, and even the size of the water bottle orifice, although the extent of their contributions is controversial [30, 31]. Some vigorous environmental stressors, such as bodily restraint, social isolation, and pain, have indeed been shown to exert physiological effects on animals and lead to anxiety-like states [30, 31]. Some authors have claimed that these tasks can be considered, at best, tests of natural preference for unlit and/or enclosed spaces because even the sensitivity to the anxiolytic effects of benzodiazepines was shown to vary among strains of mice and among anxiety tests [25, 30]. Furthermore, given that these tasks cannot be performed simultaneously with the same animal, instant sources of variation can influence them and even overrule other a priori robust effects [25]. To minimize some of these influences, several alternative anxiety-related tasks, such as the 3D maze [32], the elevated platform [33], and an integrative platform using EPM, LDB, and OF [33], have been proposed. Still, there is no ideal animal model of anxiety, and each existing test has important limitations. It seems like the best choice to study anxiety in animal models is to use multiple tests so that different facets of this same trait are assessed. Additionally, combining these tests with different pharmacological treatments, modulating their aversiveness, and testing animals with different genetic backgrounds might help obtain a clearer picture of the mechanisms underlying anxious behaviors in rodents [33].
Assuming the small overlap between the emotional aspects reflected by each different anxiety test, another relevant question is which anatomic sites each of these tasks activate. Overall, the literature suggests that the amygdala mediates fear-like behaviors to short, discrete, and proximal aversive cues, whereas the BNST mediates anxiety-like or worry-like behaviors [34], but area specificity could be also influenced by the specificity of each anxiety task. Acute activation of mPFC excitatory neurons evoked a significant decrease in anxiety-like behavior selectively in EPM but not in the OF, enhancing activation of the infralimbic, prelimbic, and cingulate subdivisions of the mPFC [34]. This was also accompanied by the activation of downstream circuits, namely the claustrum, lateral septum, bed BNST, amygdala, and hippocampus CA1 [34]. There were no changes in the nucleus accumbens, CA2, or DG [35]. IL-33 knockout mice, which naturally exhibit reduced anxiety-like behaviors (evaluated in the EPM and OF), display increased activation in the mPFC and amygdala after being submitted to the EPM [36]. Other differences between EPM and OF were observed when testing kisspeptin receptor-deleted (KISS1R-KO) male mice [37], which present behavioral alterations in the EPM but not in the OF [37]. It was suggested that GABAergic control over nigrostriatal and mesolimbic dopamine levels influenced the behavior observed in the OF test [25]. Additionally, the central amygdala, hippocampus, globo palidus, and prelimbic cortex are important for the anxiogenic effect in the OF [25]. Differences between EPM and LDB are also widely described [25, 33]. Regardless of the different zones influencing these tests, the amygdala plays a central role in anxiogenic symptoms, integrating information from cortical and thalamic sensory inputs to generate fear and anxiety-related behavioral outputs. Among its multiple subdivisions, the basolateral amygdala (BLA) and the central amygdala (CeA) are particularly important in anxiety processing [38]. More complete reviews on anxiety tasks have been published elsewhere [32,33,34].
Depression
In humans, depression is diagnosed based on a complex cluster of highly variable symptoms. In addition to depressed or irritable mood, depression includes cognitive, emotional, homeostatic, and psychomotor symptoms, and only a subset of these symptoms can objectively be measured in rodents [39]. There are many behavioral paradigms for the study of depression, and these models usually rely on behavioral readouts that can be assessed through simple behavioral tasks and that can be extrapolated as an index of the animal’s emotional state. Some models originated from the observation that stress and adverse psychosocial experiences often precede the onset or predict the recurrence of depressive episodes. The resignation latency in “despair” tests has been extensively shown to be delayed or normalized by antidepressants [40, 41]. Reductions in “positive affect” and hedonic capacity, features commonly observed in depression and that contribute to the complex construct of anhedonia, have also been modeled [42]. Finally, a trait that is often assessed as a depression-related index is the socioaffective function [43]. The 2 behavioral tests most commonly used to study depression in rodents are the forced swim test (FST) and the tail suspension test (TST), which were originally designed to predict antidepressant efficacy [44, 45]. Although it is argued by some that these tests have some face validity—the behavioral despair exhibited in response to an inescapable stressor—whether this aligns with the human condition is not clear [46, 47]. Alternative approaches modeling different traits present in depressive disorders are also used, such as tests assessing “positive affect” and hedonic capacity, features commonly observed in depression and that contribute to the complex construct of anhedonia. In fact, the preference of rodents for sweetened solutions has long been explored in science, and the first reports of decreased sucrose preference associated with depressive states date from the last century [42]. Paradigms such as the social defeat and chronic stress procedures are known to induce deficits in sucrose consumption [48]. However, it is not clear whether the form of anhedonia seen in depressed patients is the same deficit recorded in animals [46, 47, 49]. The splash test evaluates the amount of grooming performed by rodents, which can have different meanings according to the stress status of the animal. It has been shown that spray-induced grooming is negatively correlated with the duration of immobility in the FST [50] and would hence reflect an index of depressive-like behavior in the form of “self-care.” Some new tests are being developed to fill other aspects of human depression that are not covered by the aforementioned tests. The affective bias test (ABT) uses associative learning between a specific cue and a reward to test the influence of the affective state at the time of learning on the subsequent relative valuation of that reward [51]. Moreover, the judgment bias task (JBT) tests affective biases linked to decision-making behavior by evaluating the animals’ interpretation of information within the context of positive or negative associations [52]. Finally, another trait that is often studied as a depression-related index is the socioaffective function, mainly assessed through the animals’ vocalizations in different experimental settings [53].
As far as neuroanatomy is concerned, the FST shares some common neuroanatomic sites with human depression [54]. The activity of specific mPFC and mesolimbic dopaminergic circuits is important for the transition between active and passive behavioral states [54]. The ventral tegmental area (VTA) and its dopaminergic projections are also implicated in immobility in the FST [55], and pathways from the ventral hippocampus and basolateral amygdala regulate VTA dopamine neurons controlling the coping response [55]. FST also activates neurons (determined by the induction of FOS and glucose uptake) in relevant brain regions, mainly in the limbic cortical regions, lateral septum, medial amygdala, and paraventricular nucleus of the hypothalamus [54,55,56]. However, imipramine was only shown to block FOS and glucose uptake in the lateral septum but not in the cortical regions [57]. Similarly to the FST, the TST also shares some neuroanatomic similarity with human depression. TST exposure significantly activates a number of brain regions within the limbic telencephalon, hypothalamus, and brain stem, including the amygdala, but not in the hippocampus [58]. Pretreatment with antidepressants modulated neuronal activation mainly in the infralimbic cortex, lateral septal nucleus, ventrolateral preoptic nucleus, and solitary nucleus [58]. Finally, converging evidence from both rodent and human studies supports that alterations in the brain reward system underlie the anhedonia observed in rodents and humans. Mice that were exposed to social defeat developed anhedonia [59]. This phenotype was associated with increased neural activity in the prefrontal cortex, cingulate cortex, hippocampal formation, septum, amygdala, and hypothalamic nuclei [59]. The VTA and its dopaminergic projections are also relevant to sucrose preference [60], and the amygdala seems to play a central role in this behavior [61]. Additionally, the hippocampus seems to modulate sucrose preference in a SIRT6-dependent pathway [62].
Discussion
Endotoxins were described over a hundred years ago as toxins released by bacteria into their surrounding environment, but their role in the development of septic shock was only suggested halfway through the twentieth century [63]. Ever since, several studies have addressed the consequences of the exogenous administration of endotoxin via several routes—mostly intraperitoneal but also intravenous and intratracheal—and on different species, from rodents to humans. Endotoxin models are the most widely used, are the easiest to perform, and produce the greatest homogeneity among all in vivo models of sepsis [64]. In these models, the overwhelming innate immune response triggered by the administration of endotoxins has some similarities with human sepsis, although some noteworthy differences exist, such as hemodynamic alterations (low cardiac output and high vascular resistance) and the cytokines kinetics [64].
Most of the studies evaluated here induce sepsis by a single LPS injection in different mice strains (ICR, BALBc, C57BL/6, Swiss, and CD-1). Less than 15% of the studies injected LPS in rats. Additionally, in more than 95% of the studies, LPS was obtained from E. coli (0127:B8, 0111:B4, and 055:B5), and the dose range was generally from 0.1 to 0.83 mg/kg (about 90%), mostly delivered intraperitoneally (about 90%). Few studies used an LPS dose high enough to induce mortality (only 3 described mortality rate), a feature that could be expected in sepsis studies. Depressive- or anxious-like behaviors were measured mostly within the 24 h following LPS administration (in more than 90% of the studies), and this is another strong limitation if one intends to study the long-term effects of systemic inflammation. Table 1 summarizes the studies that use LPS as a model of sepsis.
The vast majority of the studies investigated both anxiety and depressive behavior 24 h after LPS administration. The effect of LPS administration in inducing anxiety- and depressive-like behavior at this time point was robust, regardless of the species (rat or mice), strain, or gender. It seems clear from these studies that a nonlethal single systemic stimulus is able to transiently induce symptoms of anxiety and depression in rodent models. The duration of these symptoms is variable, but they generally persist until the second day after LPS administration. For example, when administering a single dose of 2.5 mg/kg IP, changes in depressive-like behavior (FST) were observed up to the second day post-LPS but not after 3 or 4 days [70]. This increase in depressive-like behavior was also seen in the sucrose preference test when using an LPS dose of 0.83 mg/kg [82]. On the other hand, using a dose of 0.83 mg/kg, anxiety symptoms (EPM and LD) could be observed until 7 days after LPS administration [79]. In 2 different studies from the same group, it was possible to observe depressive symptoms at 28 days after 0.83 mg/kg LPS administration in one [92] but not in the other [93] using the same mice strain. Additionally, long-term depressive behavior was dependent on mice strain and social environment [92]. Thus, it is not possible to truly know whether these low LPS doses induce sustained alterations in the brain that mimic long-term psychiatric symptoms observed in sepsis survivors or even if the molecular alterations that occurred in this early phase are comparable to those involved in brain dysfunctions afterward.
Few studies have investigated the interaction between some comorbidities and systemic inflammation. Couch et al. (2016) [120] demonstrated that the combination of a low dose of LPS and chronic stress (CS) resulted in an enhanced depressive-like phenotype. A dose of 0.1 mg/kg LPS was not sufficient to alter EOM behavior, sucrose preference, and FST, but a prior CS potentiates LPS effects mainly by inducing 5-HT genes and IL1-β levels. The same pattern was seen using repeated very low doses of LPS (50 μg/kg) and CS. Depressive behavior combining both stimuli could be observed after 6 weeks and is related to the hippocampal kynurenine/tryptophan ratio, TNF-α levels, and astrocyte activation [143].
The largest LPS dose that was found in the literature search was LPS 10 mg/kg [65], with a reported mortality rate of 16%. Twelve weeks after a single LPS injection, animals presented anxiety-like behavior observed in the OF. This was associated with a reduced density of NeuN-immunoreactive neurons in CA1 and CA2 and in the prefrontal cortex. Additionally, acetylcholine neurons were found in parietal association and in somatosensory cortical areas. Taking a more detailed description of models that used large LPS doses, it was observed that using a dose of LPS (5 mg/kg) in C57BL/6 and a mortality of 15%, it was possible to have deficits in 3 different depressive components 28 days after LPS administration. These alterations are secondary at least to acute NRLP3 activation [68]. Using the same 5 mg/kg dose and an observed mortality of around 10%, Anderson et al. (2015) [66] observed consistent anxiogenic and depressive behaviors in different tasks (sucrose preference, TST, EPM) between 30 and 60 days after LPS. Reinforcing these findings, depressive symptoms were reversed by fluoxetine. Mechanistically, these findings were related to microglia activation in the hippocampus [66]. The only exception to these results was presented by Bossú et al. (2012) [67]. Using a dose of 5 mg/lg LPS, they did not show increased anxiety (EPM and OF), either at 7 or 280 days after LPS injection; however, the authors speculated that persistent participation of TNF-α accompanied by a contribution of IL-18 can lead to behavioral alteration. Of note, from all studies that used higher LPS doses, this was the only one that did not report any mortality and was performed in rats. Using doses of less than 5 mg/kg, long-term deficits (i.e., more than 10 days) were barely observed (Table 1).
There is a subgroup of studies that are based on neonatal sepsis. Generally, LPS was administered in a low dose (~ 50 μg/kg) at postnatal 3 to 5, and behavioral changes (both depression and anxiety) were determined at adult life [154,155,156,157,158,159,160,161,162]. Usually, adult animals more frequently developed protracted anxiety or depressive symptoms when compared to controls. Additionally, some studies injected repeated LPS doses [163,164,165,166,167,168,169,170,171,172,173,174].
The mechanisms of action of how a single LPS injection can induce acute anxiety-/depressive-like symptoms were explored. Some studies use behavioral tolls to understand how systemic inflammation interferes in brain regions and how this could induce acute anxiety-/depressive-like symptoms. For example, after a small systemic LPS injection (0.5 mg/kg), astrocytes are activated early in both the hippocampus and the amygdala and produce CXCL12 in response [130]. Microinjection of CXCL12 into the amygdala is sufficient to induce anxiety-like behaviors in mice. Both systemic LPS and amygdala CXCL12 injection-induced anxiety were blocked by an antagonist of the CXCL12 receptor (CXCR4). Additionally, the formation of quinolinic acid from tryptophan seems to be relevant because KO mice exposed to kynurenine 3-monooxygenase (KMO) or 3-hydroxyanthranilic acid dioxygenase (HAAO) are specifically protected from LPS-induced immobility in the TST [126]. Furthermore, the direct administration of 3-hydroxykynurenine, the metabolic product of KMO, caused a dose-dependent increase in depressive-like behaviors [126]. This was also true when an indoleamine 2,3-dioxygenase (IDO) inhibitor was administered [118]. Apparently, not only brain resident cells are responsible for the depressive phenotype after LPS administration because the administration of an antipolymorphonuclear antibody abolished LPS- and CLP-induced depressive behavior, brain neutrophil transmigration, and brain IL-1β levels [70, 169].
However, most of the studies have only associated different alterations related to the kynenurine pathway [79, 82, 89, 91, 94, 97, 98, 107, 118, 119, 126, 143, 146], microglial activation [66, 69, 80, 118, 119, 132, 139, 141], or astrocytic activation by the CXCL12/CXCR4 pathway [130]. The region most affected is always the hippocampus and/or the prefrontal cortex. Prager et al. (2013) report that the amygdala is affected too [151].
Most of the included studies (72%) report persistent inflammation mediated by IL6, TNF-α, and IL-1β levels as mechanisms of action. Some authors [70, 77, 78, 113, 114, 124, 129, 150] emphasize only IL-1β as the cytokine mediator of inflammation, whereas others report only TNF-α [67, 80, 90, 121, 125, 131, 132, 134, 139, 153], and still others, only IL6 [92, 93]. NF-kB is the pathway most mentioned as the route in activating cytokines after LPS injection, but only Yu et al. [74] reported that the p38/JNK pathway is activated during the LPS challenge.
Oxidative [83, 88, 101, 103, 124, 129, 138] and nitro-oxidative stress [76, 86, 87, 95, 96, 102, 106, 129] parameters are too related in some studies (12%) as the main mechanism of action of LPS injection. Besides this, alterations in neurotrophic factors have been mentioned in some studies [76, 86, 115, 134], as well as deregulation on the HPA axis [75, 92, 93, 115, 148] (Fig. 1).
Animal models of sepsis have been used in the study of the pathogenesis of neuropsychiatric disorders. LPS-induced systemic inflammation could be a useful tool to study anxiety and depressive symptoms in the context of sepsis. The CLP model consistently induces a depressive-like phenotype, making it an interesting model of the long-term mood disorders observed in human sepsis survivors. In both LPS and CLP models, basically the same molecular targets are altered: microglial activation and cytokines are frequently reported and generally affect the hippocampus; blood–brain barrier permeability is mentioned as one of the first mechanisms induced by the CLP model; besides this, oxidative stress, apoptosis, and HPA alterations are related
Another relevant tool that helps prove the concept of whether certain animal behavior is really showing anxiety- or depressive-like symptoms is the pharmacological demonstration that the anxious phenotype can be reversed using benzodiazepines or that the depressive phenotype can be reversible by an antidepressant. Unfortunately, these strategies are scarcely used in the selected articles. Additionally, when drugs are tested, they are generally administered for days before the LPS challenge and, in very few situations, after LPS administration, when the physiological alterations in the brain are already installed. However, it is not the aim of this review to describe in detail the molecular mechanisms determined from these studies.
In summary, LPS-induced systemic inflammation could be useful as a model of postsepsis anxiety and depression. One should keep in mind that anxiety disorders, depressive symptoms, and PTSD are observed in sepsis survivors [3,4,5,6], and most of the studies using LPS (mainly at doses lower than 1 mg/kg) showed 1 to 2 days of limited anxiety and depressive symptoms that resolved spontaneously. Generally, short-term septic patients present encephalopathy that is clinically characterized by impaired consciousness ranging from delirium to coma [175]. Thus, it is uncertain if an acute dose of LPS mimics what is observed in septic patients, but it is reasonable to suppose that this model could be a useful tool to understand the initial mechanisms that drive long-term psychiatric symptoms. It seems relevant when using this model that the LPS dose should be associated with some mortality (as do sepsis and septic shock) and that brain structures involved in a given behavior should be assessed. Additionally, to increase consistency, it is suggested that more than one anxious or depressive behavior should be measured and that classic drugs that reverse these behaviors should be used to double-check the specificity of the phenotype observation (i.e., benzodiazepines and antidepressants). A clear advantage of this model is the rapid timeframe between LPS administration and phenotype and molecular correlates observations. Whenever possible, long-term observation of the animal (for more than 10 days) with the use of a larger LPS dose (higher than 5 mg/kg) that elicits as 15 to 20% mortality should better mimic the clinical scenario of sepsis survivor patients and open the perspective to develop drugs to be used after the brain pathophysiological alterations have already been in course.
The cecal ligation and perforation (CLP) model was developed in the 1970s and is considered by many as the gold standard for animal models of sepsis [64]. In this model, anesthetized rats or mice are subjected to a midline laparotomy and have their cecum isolated, ligated, and perforated before having it returned to the abdominal cavity, which is then closed. This technique induces inflammatory, immune, hemodynamic, and biochemical alterations similar to human sepsis. For example, contrary to endotoxin administration, CLP induces a slower but more consistent increase in plasma cytokines that resembles human sepsis [64]. The degree of severity associated with the procedure can vary greatly according to factors such as the aseptic practices adopted, the resuscitation protocol, the site of ligation, the number of punctures, and needle size used. As a consequence, it is of great importance that the procedure is performed with high consistency and reproducibility [64].
Due to intrinsic characteristics, the acute evaluation of anxiety and depressive behavior is almost impossible in the CLP model. The influence of anesthesia, analgesia, and pain is nonnegligible, limiting the CLP model to a tool for the study of the late phases of sepsis recovery. Thus, the CLP model seems more useful for studying long-term anxiety and depressive behavior in survivors. The vast majority of CLP studies describe mortality after the procedure, generally around 30 to 50%. These experiments also systematically comprise antibiotic treatment and fluid administration. These factors support the argument that the CLP model is more closely related to human sepsis.
Within our search, only 4 studies assessed anxiety and depression after CLP in mice [176,177,178,179]. All these studies were performed in young adult male mice, mostly from the C57BL/6 strain (3 out of 4). During CLP, the number of punctures performed varied greatly (from 1 to 3), whereas the size remained constant (22G). The mortality was described for 3 of these studies and ranged from 0 to 50%. Only anxiety-related traits were evaluated in these studies, either at 10 days post-CLP (2 studies) or at a longer term (29 and 35 days, 2 studies). Only one of the studies evaluating the effect of CLP on anxious-like behavior found a significant increase in this behavior 10 days after CLP.
Behavioral assessment after CLP has been relatively more frequently performed in rats. For this group, we identified 8 studies, all performed in male Wistar rats, mostly adults (7 out of 8) [179,180,181,182,183,184,185]. The procedures always included one puncture of the same size (14G) and led to a mortality rate varying between 30 and 60%. All these studies evaluated the long-term consequences of CLP on behavior between 7 and 10 days post-CLP, and one of these studies also included a 30- and a 60-day post-CLP evaluation for both anxiety- and depression-related behaviors. All but 1 of the 7 studies that evaluated the impact of CLP on depression-related behaviors found a negative influence of this procedure up to 30 days post-CLP, regardless of the behavioral test used (FST, sucrose preference, or sweet food consumption). Among the 3 studies that evaluated anxiety-related behaviors, only 1 found an influence of CLP on this trait, with increased anxiety-related behavior in the elevated plus-maze 7 days post-CLP. Interestingly, despite the different results, all 3 studies investigating the effect of CLP on anxiety-related behaviors used readouts from the elevated plus-maze as anxiety indexes. It is noteworthy, however, that those that did not find an effect of CLP on anxiety evaluated this behavior at a longer term (from 10 to 60 days post-CLP), which might explain the variability among these results.
Overall, it seems that the CLP model consistently induces a depressive-like phenotype, although this effect needs to be confirmed in the mouse model. This effect seems to last for at least 30 days, making it an interesting model of the long-term mood disorders observed in human sepsis survivors. Although only assessed in one study, the apparent improvement of depressive-like behavior at 60 days post-CLP should be taken into account when planning long-term experiments and should be confirmed with more experiments investigating the behavioral consequences of CLP at longer terms. On the other hand, the results observed for anxiety-related behavior are not robust. Only 2 out of 7 studies found an influence of the CLP of this trait, which seems to depend greatly on the timing of the evaluation (only observed at early times) and of the behavioral task used (only observed in the EPM).
Paradigms to study depressive behavior are similar to the LPS model. Depression is generally evaluated by FST or sucrose preference and generally could demonstrate depressive behavior in sepsis survivor animals. In the vast majority of the studies during the first 10 days after CLP depression is observed in these animals, and at longer times (30-60 days), animals tend to return to sham-operated behavior (Table 2).
The differences in mainly anxiety-related behaviors are intriguing. First, in both LPS and CLP models, basically the same molecular targets are altered: Cytokines are more frequently reported and generally affect the hippocampus [169, 176,177,178, 180, 183,184,185,186,187,188]; microglial activation is reported by Gao et al. (2017); blood–brain barrier permeability is mentioned by Ozcan et al. (2015) as one of the first mechanisms induced by the CLP model; and a decrease of neurotrophic factors is related by Gao et al. (2017). Besides this, oxidative stress [181, 184, 188], apoptosis [184, 185, 187], and HPA alterations [182, 183] are related (Fig. 1).
Generally, treatments are able to completely reverse both phenotypic and molecular alterations. It seems that both models provide a time-limited dysfunction (inflammatory, metabolic, neurotransmitters) that varies secondary to characteristics of the models. A more sharp but transient increase in cytokines is achieved by LPS, contrasted with a slower, more consistent increase in CLP. These time differences are consistent with differences observed in the duration of anxiety and depressive symptoms when using these models. As for LPS models, the CLP model is useful in understanding acute molecular alterations that occur acutely in the brain, despite it being more difficult to access neurological function (see above). Probably, its great advantage is a more reliable toll to study long-term brain alterations. However, even in this context, one should interpret the data with caution. Most of the published studies suggested a time-dependent recovery from behavior alterations, despite the fact that some molecular alterations persist in the brain. So this model should probably be improved by adding some second hit to make alterations more sustained (for example, a second inflammatory hit after CLP, induce CLP in aged animals, or animals with some comorbid condition) and thus more reliable to understand the mechanisms that underlie anxiety and depression in septic survivor animals. Another limitation that should be observed is the necessity to develop a model to study PTSD in these animals.
References
Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US HOSPITALS USING CLINICAL VS CLAIMS DATA, 2009-2014. JAMA 2017;318(13):1241-1249. https://doi.org/10.1001/jama.2017.13836
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311(13):1308-1316.
Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis 2017;17(11):1180-1189.
Machado FR, Azevedo LCP. Sepsis: a threat that needs a global solution. Crit Care Med 2018;46(3): 454-459.
Prescott HC, Angus DC. Postsepsis morbidity. JAMA 2018;319(1):91.
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA 2018;319(1):62-75.
Walsh TS, Salisbury LG, Merriweather JL, Boyd JA, Griffith DM, Huby G, et al. Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: The RECOVER Randomized Clinical Trial. JAMA Intern Med 2015;175(6):901-910.
Schmidt K, Worrack S, Von Korff M, Davydow D, Brunkhorst F, Ehlert U, et al. Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: a randomized clinical trial. JAMA 2016;315(24):2703-2711.
Goddard SL, Adhikari NK. The challenging task of improving the recovery of ICU survivors. JAMA 2016; 315(24), 2671-2672.
Wade D, Als N, Bell V, Brewin C, D'Antoni D, Harrison DA, et al. Providing psychological support to people in intensive care: development and feasibility study of a nurse-led intervention to prevent acute stress and long-term morbidity. BMJ Open 2018;8(7):e021083.
Cox CE, Hough CL, Jones DM, Ungar A, Reagan W, Key MD, et al. Effects of mindfulness training programmes delivered by a self-directed mobile app and by telephone compared with an education programme for survivors of critical illness: a pilot randomised clinical trial. Thorax 2019;74(1):33-42.
Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Della Giustina A, et al. Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol 2019;56(1):186-251.
Barichello T, Generoso JS, Goularte JA, Collodel A, Pitcher MR, Simões LR, et al. Does infection-induced immune activation contribute to dementia? Aging Dis 2015;6(5):342-348.
Bozza FA, D'Avila JC, Ritter C, Sonneville R, Sharshar T, Dal-Pizzol F. Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 2013;39 Suppl 1:10-16.
Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med 2015; 3(1):61-69.
Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23-29.
Rabiee A, Nikayin S, Hashem MD, Huang M, Dinglas VD, Bienvenu OJ, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med 2016; 44(9):1744-1753.
Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med 2015; 43(5):1121-1129.
Riegel B, Huang L, Mikkelsen ME, Kutney-Lee A, Hanlon AL, Murtaugh CM, Bowles KH. Early post-intensive care syndrome among older adult sepsis survivors receiving home care. J Am Geriatr Soc 2019;67(3):520-526.
Thompson K, Taylor C, Jan S et al. Health-related outcomes of critically ill patients with and without sepsis. Intensive Care Med. 2018; 44:1249–1257.
Huang CY, Daniels R, Lembo A, Hartog C, O'Brien J, Heymann T, et al. Sepsis Survivors Engagement Project (SSEP). Life after sepsis: an international survey of survivors to understand the post-sepsis syndrome. Int J Qual Health Care 2019;31(3):191-198.
Haines D, Hild J, He J, Stun L, Ballew A, Green JL, et al. A retrospective pilot study of de novo antidepressant medication initiation in intensive care unit patients and post-ICU depression,. Crit Care Res Pract. 2017; 2017:5804860.
Harro J. Animal models of depression: pros and cons. Cell Tissue Res 2018. https://doi.org/10.1007/s00441-018-2973-0.
Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019; 99:101-116. https://doi.org/10.1016/j.neubiorev.2018.12.002.
Mohammad F, Ho J, Woo JH, Lim CL, Poon DJJ, Lamba B, et al. Concordance and incongruence in preclinical anxiety models: Systematic review and meta-analyses. Neurosci Biobehav Rev 2016; 68:504-529.
Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015; 16(6), 317-331.
Schmidt CK, Khalid S, Loukas M, Tubbs RS. Neuroanatomy of anxiety: a brief review. Cureus 2018; 10(1):e2055.
Baldwin DS, Hou R, Gordon R, Huneke NT, Garner M. Pharmacotherapy in generalized anxiety disorder: novel experimental medicine models and emerging drug targets. CNS Drugs 2017; 31(4), 307-317.
Ennaceur A, Chazot PL. Preclinical animal anxiety research - flaws and prejudices. Pharmacol Res Perspect 2016; 4(2), e00223.
Bourin M, Petit-Demoulière B, Dhonnchadha B.N, Hascöet M. Animal models of anxiety in mice. Fundam Clin Pharmacol 2007; 21(6), 567-574.
Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol 2003; 463(1-3):55-65.
Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. Models of anxiety: responses of mice to novelty and open spaces in a 3D maze. Behav Brain Res 2006; 174(1), 9-38.
Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 2008; 29(10), 493-498.
Pati S, Sood A, Mukhopadhyay S, Vaidya VA. Acute pharmacogenetic activation of medial prefrontal cortex excitatory neurons regulates anxiety-like behaviour. J Biosci 2018; 43(1), 85-95.
Ishikawa J, Nishimura R, Ishikawa A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur J Neurosci 2015; 41(4), 442-453.
Dohi E, Choi EY, Rose IVL, Murata AS, Chow S, Niwa M, et al. Behavioral changes in mice lacking interleukin-33. eNeuro. 2017;4(6):ENEURO.0147-17.2017.
Delmas S, Porteous R, Bergin DH, Herbison AE. Altered aspects of anxiety-related behavior in kisspeptin receptor-deleted male mice. Sci Rep 2018; 8(1), 2794.
Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015;527(7577):179-185.
Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010;13(10), 1161–1169.
Hales CA, Stuart SA, Anderson MH, Robinson ESJ. Behavioural and computational methods reveal differential effects for how delayed and rapid onset antidepressants effect decision making in rats. Br J Pharmacol 2014; 171(20), 4524–4538.
Ma L, Demin KA, Kolesnikova TO, Khatsko SL, Zhu X, Yuan X, et al. Animal inflammation-based models of depression and their application to drug discovery. Expert Opin Drug Discovery 2017;12(10):995-1009.
Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 2014; 10:393-423.
Robinson ESJ. Translational new approaches for investigating mood disorders in rodents and what they may reveal about the underlying neurobiology of major depressive disorder. Philos Trans R Soc Lond Ser B Biol Sci 2018; 373:1742.
Yin X, Guven N, Dietis N. Stress-based animal models of depression: do we actually know what we are doing? Brain Res 2016; 1652, 30-42.
Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Braz J Psychiatry 2013; 35, S112-120.
Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior, ACS Chem Neurosci 2017; 8(5), 955-960.
Molendijk ML, de Kloet ER. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015; 62, 389-391.
Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav 1996; 60(6), 1481-1484.
Holmes PV. Rodent models of depression: reexamining validity without anthropomorphic inference. Crit Rev Neurobiol 2003; 15(2):143-174.
Hu C, Luo Y, Wang H, Kuang S, Liang G, Yang Y, Mai S, Yang J. Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS One 2017;12(9):e0185129.
Hinchcliffe JK, Stuart SA, Mendl M, Robinson ESJ. Further validation of the affective bias test for predicting antidepressant and pro-depressant risk: effects of pharmacological and social manipulations in male and female rats. Psychopharmacology 2017; 234(20), 3105-3116.
Jones S, Paul ES, Dayan P, Robinson ESJ, Mendl M. Pavlovian influences on learning differ between rats and mice in a counter-balanced Go/NoGo judgement bias task. Behav Brain Res 2017; 331, 214-224.
Wöhr M, van Gaalen MM, Schwarting RK. Affective communication in rodents: serotonin and its modulating role in ultrasonic vocalizations. Behav Pharmacol 2015; 26(6): 506-521.
Bruder GE., Stewart JW, McGrath PJ. Right brain, left brain in depressive disorders: Clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neurosci Biobehav Rev 2017; 78: 178-191.
Donofry SD, Roecklein KA, Wildes JE, Miller MA, Erickson KI. Alterations in emotion generation and regulation neurocircuitry in depression and eating disorders: a comparative review of structural and functional neuroimaging studies. Neurosci Biobehav Rev 2016; 68, 911-927.
Nam H, Kerman IA. A2 noradrenergic neurons regulate forced swim test immobility. Physiol Behav 2016; 165:339-349.
Yanagida S, Motomura K, Ohashi A, Hiraoka K, Miura T, Kanba S. Effect of acute imipramine administration on the pattern of forced swim-induced c-Fos expression in the mouse brain. Neurosci Lett 2016;629:119-124.
Hiraoka K, Motomura K, Yanagida S, Ohashi A, Ishisaka-Furuno N, Kanba S. Pattern of c-Fos expression induced by tail suspension test in the mouse brain. Heliyon 3(6), e00316 (2017).
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry 2014; 76(7), 542-549.
Shibata R, Kameishi M, Kondoh T, Torii K. Bilateral dopaminergic lesions in the ventral tegmental area of rats influence sucrose intake, but not umami and amino acid intake. Physiol Behav 2009; 96(4-5), 667-674.
Yasoshima Y, Yoshizawa H, Shimura T, Miyamoto T. The basolateral nucleus of the amygdala mediates caloric sugar preference over a non-caloric sweetener in mice. Neuroscience 2015; 291, 203-215.
Mao Q, Gong X, Zhou C, Tu Z, Zhao L, Wang L, et al. Up-regulation of SIRT6 in the hippocampus induced rats with depression-like behavior via the block Akt/GSK3β signaling pathway. Behav Brain Res 2017;323:38-46.
Vincent JL, Abraham E. The last 100 years of sepsis. Am J Respir Crit Care Med 2006; 173(3), 256-263.
Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med 2009; 37(1 Suppl), S30-37.
Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol 2007;204(2):733-40.
Anderson ST, Commins S, Moynagh PN, Coogan AN. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav Immun 2015; 43, 98-109.
Bossù P, Cutuli D, Palladino I, Caporali P, Angelucci F, Laricchiuta D, et al. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J Neuroinflammation 2012;9:101.
Zhu W, Cao FS, Feng J, Chen HW, Wan JR, Lu Q, Wang J. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience 2017; 343:77-84.
Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, Shirawachi S, Asano S, Aizawa H, Yamawaki S, Kanematsu T, Akagi H. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res 2018;1680:13-38.
Aguilar-Valles A, Kim J, Jung S, Woodside B, Luheshi GN. Role of brain transmigrating neutrophils in depression-like behavior during systemic infection. Mol Psychiatry 2014; 19(5),599-606.
Sekio M, Seki K. Lipopolysaccharide-induced depressive-like behavior is associated with α1-adrenoceptor dependent downregulation of the membrane GluR1 subunit in the mouse medial prefrontal cortex and ventral tegmental area. Int J Neuropsychopharmacol. 2014; 18(1), pyu005.
Jeon SA, Lee E, Hwang I, Han B, Park S, Son S, et al. NLRP3 inflammasome contributes to lipopolysaccharide-induced depressive-like behaviors via indoleamine 2,3-dioxygenase induction. Int J Neuropsychopharmacol 2017;20(11):896-906.
Ismail N, Kumlin AM, Blaustein JD. A pubertal immune challenge alters the antidepressant-like effects of chronic estradiol treatment in inbred and outbred adult female mice. Neuroscience 2013; 249, 43-52.
Yu H, Zou Z, Zhang X, Peng W, Chen C, Ye Y, et al. Inhibition of phosphodiesterase 4 by FCPR03 alleviates lipopolysaccharide-induced depressive-like behaviors in mice: involvement of p38 and JNK signaling pathways. Int J Mol Sci 2018;19(2):513.
Ming Z, Sawicki G, Bekar LK. Acute systemic LPS-mediated inflammation induces lasting changes in mouse cortical neuromodulation and behavior. Neurosci Lett 590, 96-100 (2015).
Sriram CS, Jangra A, Gurjar SS, Mohan P, Bezbaruah BK. Edaravone abrogates LPS-induced behavioral anomalies, neuroinflammation and PARP-1. Physiol Behav 2016; 154, 135-144.
Zhang F, Fu Y, Zhou X, Pan W, Shi Y, Wang M, et al. Depression-like behaviors and heme oxygenase-1 are regulated by lycopene in lipopolysaccharide-induced neuroinflammation. J Neuroimmunol 2016; 298:1-8.
Li M, Li C, Yu H, Cai X, Shen X, Sun X, et al. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice J Neuroinflammation 2017;14(1):190.
Bhatt S, Mahesh R, Devadoss T, Jindal A. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (4-benzylpiperazin-1-yl) (3-methoxyquinoxalin-2-yl) methanone (6g) on lipopolysaccharide-induced anxiety models in mice. J Basic Clin Physiol Pharmacol 2017; 28(2), 101-106.
Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Baune BT. Effects of centrally administered etanercept on behavior, microglia, and astrocytes in mice following a peripheral immune challenge. Neuropsychopharmacology 2015;40(2):502-12.
Campos AC, Rocha NP, Nicoli JR, Vieira LQ, Teixeira MM, Teixeira AL. Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav Brain Res 2016;312:186-94.
Frenois F, et al: Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32(5), 516-531 (2007).
Gawali NB, Bulani VD, Chowdhury AA, Deshpande PS, Nagmoti DM, Juvekar AR. Agmatine ameliorates lipopolysaccharide induced depressive-like behaviour in mice by targeting the underlying inflammatory and oxido-nitrosative mediators. Pharmacol Biochem Behav 2016;149:1-8.
Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, et al. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol 2015;768:49-57.
Hall S, Arora D, Anoopkumar-Dukie S, Grant GD. Effect Of Coffee In Lipopolysaccharide-Induced Indoleamine 2,3-Dioxygenase Activation And Depressive-Like Behavior In Mice. J Agric Food Chem 2016; 64(46), 8745-8754.
Jangra A, Sriram CS, Lahkar M. Lipopolysaccharide-induced behavioral alterations are alleviated by sodium phenylbutyrate via attenuation of oxidative stress and neuroinflammatory cascade. Inflammation 2016; 39(4),1441-1452.
Jangra A, Lukhi MM, Sulakhiya K, Baruah CC, Lahkar M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur J Pharmacol 2014; 740, 337-345.
Jiang W, Chen Q, Li P, Lu Q, Pei X, Sun Y, Wang G, Hao K. Magnesium isoglycyrrhizinate attenuates lipopolysaccharide-induced depressive-like behavior in mice. Biomed Pharmacother 2017;86:177-184.
Kang A, Xie T, Zhu D, Shan J, Di L, Zheng X. Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J Agric Food Chem 2017;65(32):6861-6869.
Lawson MA, McCusker RH, Kelley KW. Interleukin-1 beta converting enzyme is necessary for development of depression-like behavior following intracerebroventricular administration of lipopolysaccharide to mice. J Neuroinflammation 2013; 10:54.
O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009;14(5):511-22.
Painsipp E, Köfer M.J, Sinner F, Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS One 2011; 6(6), e20719.
Painsipp E, Herzog H, Holzer P. Evidence from knockout mice that neuropeptide-Y Y2 and Y4 receptor signalling prevents long-term depression-like behaviour caused by immune challenge. J Psychopharmacol 2010; 24(10), 1551-1560.
Park SE, Lawson M, Dantzer R, Kelley KW, McCusker RH. Insulin-like growth factor-I peptides act centrally to decrease depression-like behavior of mice treated intraperitoneally with lipopolysaccharide. J Neuroinflammation 2011; 8, 179.
Sulakhiya K, Keshavlal GP, Bezbaruah BB, Dwivedi S, Gurjar SS, Munde N, et al. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett 2016;611:106-11.
Sulakhiya K, Kumar P, Jangra A, Dwivedi S, Hazarika NK, Baruah CC, Lahkar M. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur J Pharmacol 2014;744:124-31.
Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O'Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav 2012; 62(3), 202-209.
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013;38(9):1609-16.
Wang Z, Li W, Chen J, Shi H, Zhao M, You H, et al. Proteomic analysis reveals energy metabolic dysfunction and neurogenesis in the prefrontal cortex of a lipopolysaccharide-induced mouse model of depression. Mol Med Rep 2016;13(2):1813-20.
Wang Z, Zhang Q, Yuan L, Wang S, Liu L, Yang X, et al. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav Brain Res 2014;274:282-90.
Wu Y, Fu Y, Rao C, Li W, Liang Z, Zhou C, et al. Metabolomic analysis reveals metabolic disturbances in the prefrontal cortex of the lipopolysaccharide-induced mouse model of depression. Behav Brain Res 2016;308:115-27.
Yang R, Wang P, Chen Z, Hu W, Gong Y, Zhang W, Huang C. WY-14643, a selective agonist of peroxisome proliferator-activated receptor-α, ameliorates lipopolysaccharide-induced depressive-like behaviors by preventing neuroinflammation and oxido-nitrosative stress in mice. Pharmacol Biochem Behav 2017;153:97-104.
Yu X, Jiang X, Zhang X, Chen Z, Xu L, Chen L, et al. The effects of fisetin on lipopolysaccharide-induced depressive-like behavior in mice. Metab Brain Dis 2016;31(5):1011-21.
Zhu L, Nang C, Luo F, Pan H, Zhang K, Liu J, et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol Behav 2016;163:184-192.
Jiang X, Liu J, Lin Q, Mao K, Tian F, Jing C, et al. Proanthocyanidin prevents lipopolysaccharide-induced depressive-like behavior in mice via neuroinflammatory pathway. Brain Res Bull. 2017;135:40-46.
Barua CC, Haloi P, Saikia B, Sulakhiya K, Pathak DC, Tamuli S, et al. Zanthoxylum alatum abrogates lipopolysaccharide-induced depression-like behaviours in mice by modulating neuroinflammation and monoamine neurotransmitters in the hippocampus. Pharm Biol 2018;56(1):245-252.
Hao K, Qi Q, Hao H, Wang G, Chen Y, Liang Y, Xie L. The pharmacokinetic-pharmacodynamic model of azithromycin for lipopolysaccharide-induced depressive-like behavior in mice. PLoS One 2013;8(1):e54981.
Medeiros IU, Ruzza C, Asth L, Guerrini R, Romão PR, Gavioli EC, Calo G. Blockade of nociceptin/orphanin FQ receptor signaling reverses LPS-induced depressive-like behavior in mice. Peptides 2015;72:95-103.
Wu TY, Liu L, Zhang W, Zhang Y, Liu YZ, Shen XL, et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J Psychiatr Res 2015; 64:99-106.
Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther 2014;20(2):119-24.
Deyama S, Shimoda K, Ikeda H, Fukuda H, Shuto S, Minami M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J Pharmacol Sci 2018;138(1):86-88.
Deyama S, Shimoda K, Suzuki H, Ishikawa Y, Ishimura K, Fukuda H, et al. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology 2018;235(1):329-336.
Savignac HM, Couch Y, Stratford M, Bannerman DM, Tzortzis G, Anthony DC, Burnet PWJ. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav Immun 2016; 52:120-131.
Fischer CW, Elfving B, Lund S, Wegener G. Behavioral and systemic consequences of long-term inflammatory challenge. J Neuroimmunol 2015; 288, 40-46.
Adzic M, Djordjevic J, Mitic M, Brkic Z, Lukic I, Radojcic M. The contribution of hypothalamic neuroendocrine, neuroplastic and neuroinflammatory processes to lipopolysaccharide-induced depressive-like behaviour in female and male rats: Involvement of glucocorticoid receptor and C/EBP-β. Behav Brain Res 2015;291:130-139.
Araki R, Hiraki Y, Nishida S, Inatomi Y, Yabe T. Gomisin ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J Pharmacol Sci 2016; 132(2), 138-144.
Bassi GS, Kanashiro A, Santin FM, de Souza GE, Nobre MJ, Coimbra NC. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin Pharmacol Toxicol 2012;110(4):359-69.
Corona AW, Norden DM, Skendelas JP, Huang Y, O'Connor JC, Lawson M, et al. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun 2013;31:134-42.
Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation 2010;7:93.
Couch Y, Trofimov A, Markova N, Nikolenko V, Steinbusch HW, Chekhonin V, et al. Low-dose lipopolysaccharide (LPS) inhibits aggressive and augments depressive behaviours in a chronic mild stress model in mice. J Neuroinflammation 2016;13(1):108.
Dong C, Zhang JC, Yao W, Ren Q, Yang C, Ma M, et al. Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 2016; 144:7-12.
Ji WW, Wang SY, Ma ZQ, Li RP, Li SS, Xue JS, et al. Effects of perillaldehyde on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 2014; 116:1-8.
Li R, Zhao D, Qu R, Fu Q, Ma S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci Lett 2015; 594, 17-22.
Mello BS, Monte AS, McIntyre RS, Soczynska JK, Custódio CS, Cordeiro RC, et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 2013;47(10):1521-9.
Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 2013; 103(4), 853-859.
Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O'Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 2016;6(10):e918.
Su Q, et al. Protective effect of liquiritigenin on depressive-like behavior in mice after lipopolysaccharide administration. Psychiatry Res 240, 131-136 (2016).
Tao W, et al. Paeonol attenuates lipopolysaccharide-induced depressive-like behavior in mice. Psychiatry Res 238, 116-121 (2016).
Tomaz VS, et al. Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 268, 236-246 (2014).
Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang Q, et al. Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun 2016;56:352-62.
Yao W, et al. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 136, 7-12 (2015).
Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al: Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutr Biochem 39, 134-144 (2017).
Zhang K, et al. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci Lett 613, 60-65 (2016).
Zhang JC, et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014; 18(4):pyu077. https://doi.org/10.1093/ijnp/pyu077.
Zhe Q, Sulei W, Weiwei T, Hongyan L, Jianwei W. Effects of Jiaotaiwan on depressive-like behavior in mice after lipopolysaccharide administration. Metab Brain Dis 32(2), 415-426 (2017).
Zhu L, et al. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci Lett 606, 1-6 (2015).
Li DD, et al. Antidepressant-like effect of zileuton is accompanied by hippocampal neuroinflammation reduction and CRBE/BDNF upregulation in lipopolysaccharide-challenged mice. J Affect Disord. 227, 672-680 (2018).
Li C, et al. Neuropeptide VGF C-terminal peptide TLQP-62 alleviates lipopolysaccharide-induced memory deficits and anxiety-like and depression-like behaviors in mice: the role of BDNF/TrkB signaling. ACS Chem Neurosci. 8(9), 2005-2018 (2017).
Viana AF, et al. Kinin B1 receptors mediate depression-like behavior response in stressed mice treated with systemic E. coli lipopolysaccharide. J Neuroinflammation. 7, 98 (2010).
Godbout JP, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology 33(10), 2341-2351 (2008).
Henry CJ, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 5, 15 (2008).
Yang J, Qi F, Yao Z. Neonatal Bacillus Calmette-Guérin vaccination alleviates lipopolysaccharide-induced neurobehavioral impairments and neuroinflammation in adult mice. Mol Med Rep 14(2), 1574-1586 (2016).
Elgarf AS, Aboul-Fotouh S, Abd-Alkhalek HA, El Tabbal M, Hassan AN, Kassim SK, et al. Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline. Pharmacol Biochem Behav 2014;126:152-62.
An L, et al. Effects of the total flavonoid extract of Xiaobuxin-Tang on depression-like behavior induced by lipopolysaccharide and proinflammatory cytokine levels in mice. J Ethnopharmacol 163, 83-87 (2015).
Avitsur R, et al. Escitalopram or novel herbal treatments differentially alter cytokine and behavioral responses to immune challenge. J Neuroimmunol 309, 111-118 (2017).
Dinel AL, et al. Lipopolysaccharide-induced brain activation of the indoleamine 2,3-dioxygenase and depressive-like behavior are impaired in a mouse model of metabolic syndrome. Psychoneuroendocrinology 40, 48-59 (2014).
Haba R, et al. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: Possible role of activation of the central amygdala. Behav Brain Res 228(2), 423-431 (2012).
Renault J, Aubert A. Immunity and emotions: lipopolysaccharide increases defensive behaviours and potentiates despair in mice. Brain Behav Immun 20(6), 517-526 (2006).
Wang D, et al. Chronic blockade of glucocorticoid receptors by RU486 enhances lipopolysaccharide-induced depressive-like behaviour and cytokine production in rats. Brain Behav Immun 25(4), 706-714 (2011).
Lieberknecht V, et al. Antidepressant-like effect of pramipexole in an inflammatory model of depression. Behav Brain Res 320, 365-373 (2017).
Prager G, Hadamitzky M, Engler A, Doenlen R, Wirth T, Pacheco-López G, et al: Amygdaloid signature of peripheral immune activation by bacterial lipopolysaccharide or staphylococcal enterotoxin B. J NeuroImmune Pharmacol 8(1), 42-50 (2013).
Pitychoutis PM, Nakamura K, Tsonis PA, Papadopoulou-Daifoti Z. Neurochemical and behavioral alterations in an inflammatory model of depression: sex differences exposed. Neuroscience159(4), 1216-1232 (2009).
Yeh KY, et al. Effect of Ginkgo biloba extract on lipopolysaccharide-induced anhedonic depressive-like behavior in male rats. Phytother Res 29(2), 260-266 (2015).
Bukhari SHF, Clark OE, Williamson LL. Maternal high fructose diet and neonatal immune challenge alter offspring anxiety-like behavior and inflammation across the lifespan. Life Sci 2018; 197, 114-121.
Tishkina A, Stepanichev M, Kudryashova I, Freiman S, Onufriev M, Lazareva N, et al. Neonatal proinflammatory challenge in male Wistar rats: effects on behavior, synaptic plasticity, and adrenocortical stress response. Behav Brain Res 2016; 304: 1-10.
MacRae M, Macrina T, Khoury A, Migliore M.M, Kentner AC. Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neuroscience 2015; 298, 455-466.
Doosti MH, Bakhtiari A, Zare P, Amani M, Majidi-Zolbanin N, Babri S, Salari AA. Impacts of early intervention with fluoxetine following early neonatal immune activation on depression-like behaviors and body weight in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 2013; 43:55-65.
-Tenk CM, Kavaliers M, Ossenkopp KP. Neonatal treatment with lipopolysaccharide differentially affects adult anxiety responses in the light-dark test and taste neophobia test in male and female rats. Int J Dev Neurosci 2013; 31(3), 171-180.
Sominsky L, Walker AK, Ong LK, Tynan RJ, Walker FR, Hodgson DM. Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behav Brain Res 2012; 226(1):351-6.
Lucchina L, Carola V, Pitossi F, Depino AM. Evaluating the interaction between early postnatal inflammation and maternal care in the programming of adult anxiety and depression-related behaviors. Behav Brain Res 2010; 213(1), 56-65.
Rico JL, Ferraz DB, Ramalho-Pinto FJ, Morato S. Neonatal exposure to LPS leads to heightened exploratory activity in adolescent rats. Behav Brain Res 2010; 215(1), 102-109.
Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology 2009;34(10):1515-25.
d'Avila JC, Siqueira LD, Mazeraud A, Azevedo EP, Foguel D, Castro-Faria-Neto HC, et al. Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J Neuroinflammation 2018;15(1):28.
Duda W, Kubera M, Kreiner G, Curzytek K, Detka J, Głombik K, et al.Suppression of pro-inflammatory cytokine expression and lack of anti-depressant-like effect of fluoxetine in lipopolysaccharide-treated old female mice. Int Immunopharmacol 2017; 48:35-42.
Moraes MM, Galvão MC, Cabral D, Coelho CP, Queiroz-Hazarbassanov N, Martins MF, et al. Propentofylline prevents sickness behavior and depressive-like behavior induced by lipopolysaccharide in rats via neuroinflammatory pathway. PLoS One 2017;12(1):e0169446.
Krishna S, Dodd CA, Filipov NM. Behavioral and monoamine perturbations in adult male mice with chronic inflammation induced by repeated peripheral lipopolysaccharide administration. Behav Brain Res 2016; 302, 279-290.
Liu L, Zhang Q, Cai Y, Sun D, He X, Wang L, et al. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget 2016;7(35):56045-56059.
Guo J, Lin P, Zhao X, Zhang J, Wei X, Wang Q, Wang C. Etazolate abrogates the lipopolysaccharide (LPS)-induced downregulation of the cAMP/pCREB/BDNF signaling, neuroinflammatory response and depressive-like behavior in mice. Neuroscience 2014;263:1-14.
He H, Geng T, Chen P, et al. NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci Rep 2016; 6, 27711.
Kubera M, Curzytek K, Duda W, Leskiewicz M, Basta-Kaim A, Budziszewska B, et al. A new animal model of (chronic) depression induced by repeated and intermittent lipopolysaccharide administration for 4 months. Brain Behav Immun 2013;31:96-104.
Bay-Richter C, Hallberg L, Ventorp F, Janelidze S, Brundin L. Aldosterone synergizes with peripheral inflammation to induce brain IL-1β expression and depressive-like effects. Cytokine 2012; 60(3), 749-754.
Wang Y, Cui XL, Liu YF, Gao F, Wei D, Li XW, et al. LPS inhibits the effects of fluoxetine on depression-like behavior and hippocampal neurogenesis in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 2011;35(8):1831-5.
Berkiks I, Boulbaroud S, Garcia-Segura LM, Mesfioui A, Ouichou A, Mouden S, et al. Thymelaea lythroides extract attenuates microglial activation and depressive-like behavior in LPS-induced inflammation in adult male rats. Biomed Pharmacother. 2018; 99:655-663.
Yang M, Dang R, Xu P, Guo Y, Han W, Liao D, Jiang P. Dl-3-n-Butylphthalide improves lipopolysaccharide-induced depressive-like behavior in rats: involvement of Nrf2 and NF-κB pathways. Psychopharmacology 2018;235(9):2573-2585.
Heming N, Mazeraud A, Verdonk F, Bozza FA, Chrétien F, Sharshar T. Neuroanatomy of sepsis-associated encephalopathy. Crit Care 2017;21(1):65.
Gao R, Ji MH, Gao DP, Yang RH, Zhang SG, Yang JJ, Shen JC. Neuroinflammation-induced downregulation of hippocampal neuregulin 1-ErbB4 signaling in the parvalbumin interneurons might contribute to cognitive impairment in a mouse model of sepsis-associated encephalopathy. Inflammation 2017;40(2):387-400.
Wu J, Dong L, Zhang M, Jia M, Zhang G, Qiu L, et al. Class I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathy. Neurochem Res 2013;38(11):2440-9.
Calsavara AC, Rodrigues DH, Miranda AS, Costa PA, Lima CX, Vilela MC, et al. Late anxiety-like behavior and neuroinflammation in mice subjected to sublethal polymicrobial sepsis. Neurotox Res 2013;24(2):103-8.
Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med 2012;18(1):930-7.
Leite FB, Prediger RD, Silva MV, de Sousa JB, Carneiro FP, Gasbarri A, et al. Role of nicotine on cognitive and behavioral deficits in sepsis-surviving rats. Brain Res 2013;1507:74-82.
Petronilho F, Périco SR, Vuolo F, Mina F, Constantino L, Comim CM, Quevedo J, Souza DO, Dal-Pizzol F. Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun 2012;26(6):904-10.
Cassol OJ Jr, Comim CM, Petronilho F, Constantino LS, Streck EL, Quevedo J, Dal-Pizzol F. Low dose dexamethasone reverses depressive-like parameters and memory impairment in rats submitted to sepsis. Neurosci Lett 2010;473(2):126-30.
Comim CM, Cassol OJ Jr, Constantino LC, Petronilho F, Constantino LS, Stertz L, Kapczinski F, Barichello T, Quevedo J, Dal-Pizzol F. Depressive-like parameters in sepsis survivor rats. Neurotox Res 2010;17(3):279-86.
Tuon L, Comim CM, Petronilho F, Barichello T, Izquierdo I, Quevedo J, Dal-Pizzol F. Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med 2008;34(9):1724-31.
Tuon L, Comim CM, Antunes MM, Constantino LS, Machado RA, Izquierdo I, Quevedo J, Dal-Pizzol F. Imipramine reverses the depressive symptoms in sepsis survivor rats. Intensive Care Med 2007;33(12):2165-7.
Ozcan PE, et al. Effects of intravenous immunoglobulin therapy on behavior deficits and functions in sepsis model. Ann Intensive Care 5(1), 62 (2015).
Barichello T, Martins MR, Reinke A, Constantino LS, Machado RA, Valvassori SS, Moreira JC, Quevedo J, Dal-Pizzol F. Behavioral deficits in sepsis-surviving rats induced by cecal ligation and perforation. Braz J Med Biol Res 2007;40(6):831-7.
Steckert AV, Dominguini D, Michels M, Abelaira HM, Tomaz DB, Sonai B, et al. The impact of chronic mild stress on long-term depressive behavior in rats which have survived sepsis. J Psychiatr Res. 2017; 94:47-53.
Acknowledgments
CNPq.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
CNPq. The funders had no role in the design, analysis, write-up, or decision to submit for publication.
Author information
Authors and Affiliations
Contributions
Study conception: FD-P, GFM, CR, and TS; study design: FD-P and GFM; study/research conduct: FD-P, GFM, MM, AM, FAB, and CR; drafting of report: FD-P, GFM, MM, CR, FAB, and TS. All of the study authors had full access to all of the data in the study, and can take responsibility for the integrity of the data and the accuracy of the analyses. All of the authors reviewed the report for important intellectual content and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1899 kb)
Rights and permissions
About this article
Cite this article
Dal-Pizzol, F., de Medeiros, G.F., Michels, M. et al. What Animal Models Can Tell Us About Long-Term Psychiatric Symptoms in Sepsis Survivors: a Systematic Review. Neurotherapeutics 18, 1393–1413 (2021). https://doi.org/10.1007/s13311-020-00981-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-020-00981-9