Abstract
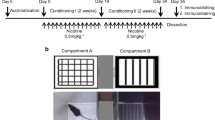
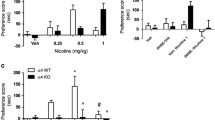
Nicotine causes psychological dependence through its interactions with nicotinic acetylcholine receptors in the brain. We previously demonstrated that fatty acid-binding protein 3 (FABP3) colocalizes with dopamine D2 receptors (D2Rs) in the dorsal striatum, and FABP3 deficiency leads to impaired D2R function. Moreover, D2R null mice do not exhibit increased nicotine-induced conditioned place preference (CPP) following chronic nicotine administration. To investigate the role of FABP3 in nicotine-induced CPP, FABP3 knockout (FABP3−/−) mice were evaluated using a CPP apparatus following consecutive nicotine administration (0.5 mg/kg) for 14 days. Importantly, nicotine-induced CPP was suppressed in the conditioning, withdrawal, and relapse phases in FABP3−/− mice. To resolve the mechanisms underlying impaired nicotine-induced CPP in these mice, we assessed c-Fos expression and Ca2+/calmodulin-dependent protein kinase II (CaMKII) and extracellular signal-regulated kinase (ERK) signaling in both dopamine D1 receptor (D1R)- and D2R-positive neurons in the nucleus accumbens (NAc). Notably, 64% of dopamine receptor-positive neurons in the mouse NAc expressed both D1R and D2R. Impaired nicotine-induced CPP was correlated with lack of responsiveness of both CaMKII and ERK phosphorylation. The number of D2R-positive neurons was increased in FABP3−/− mice, while the number of D1R-positive neurons and the responsiveness of c-Fos expression to nicotine were decreased. The aberrant c-Fos expression was closely correlated with CaMKII but not ERK phosphorylation levels in the NAc of FABP3−/− mice. Taken together, these results indicate that impaired D2R signaling due to lack of FABP3 may affect D1R and c-Fos signaling and underlie nicotine-induced CPP behaviors.






Similar content being viewed by others
Data Availability
There is no availability of data and material. All data were included in the manuscript.
References
Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, Rose JE, Levin ED (2014) Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav 120:103–108. https://doi.org/10.1016/j.pbb.2014.02.011
Rose JE, Corrigall WA (1997) Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology 130(1):28–40. https://doi.org/10.1007/s002130050209
Brazell MP, Mitchell SN, Joseph MH, Gray JA (1990) Acute administration of nicotine increases the in vivo extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid and ascorbic acid preferentially in the nucleus accumbens of the rat: comparison with caudate-putamen. Neuropharmacology 29(12):1177–1185. https://doi.org/10.1016/0028-3908(90)90042-p
Calabresi P, Lacey MG, North RA (1989) Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol 98(1):135–140. https://doi.org/10.1111/j.1476-5381.1989.tb16873.x
Koob GF, Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science 242(4879):715–723. https://doi.org/10.1126/science.2903550
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78(1):189–225. https://doi.org/10.1152/physrev.1998.78.1.189
Cahill E, Salery M, Vanhoutte P, Caboche J (2014) Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse. Front Pharmacol 4:172. https://doi.org/10.3389/fphar.2013.00172
Haghparast A, Omranifard A, Arezoomandan R, Ghalandari-Shamami M, Taslimi Z, Vafaei AA, Rashidy-Pour A (2013) Involvement of dopaminergic receptors of the rat nucleus accumbens in decreasing the conditioned place preference induced by lateral hypothalamus stimulation. Neurosci Lett 556:10–14. https://doi.org/10.1016/j.neulet.2013.09.062
Esmaeili MH, Kermani M, Parvishan A, Haghparast A (2012) Role of D1/D2 dopamine receptors in the CA1 region of the rat hippocampus in the rewarding effects of morphine administered into the ventral tegmental area. Behav Brain Res 231(1):111–115. https://doi.org/10.1016/j.bbr.2012.02.050
Wilar G, Shinoda Y, Sasaoka T, Fukunaga K (2019) Crucial role of dopamine D2 receptor signaling in nicotine-induced conditioned place preference. Mol Neurobiol 56(12):7911–7928. https://doi.org/10.1007/s12035-019-1635-x
Grieder TE, Sellings LH, Vargas-Perez H, Ting AKR, Siu EC, Tyndale RF, van der Kooy D (2010) Dopaminergic signaling mediates the motivational response underlying the opponent process to chronic but not acute nicotine. Neuropsychopharmacology 35(4):943–954. https://doi.org/10.1038/npp.2009.198
Kutlu MG, Burke D, Slade S, Hall BJ, Rose JE, Levin ED (2013) Role of insular cortex D(1) and D(2) dopamine receptors in nicotine self-administration in rats. Behav Brain Res 256:273–278. https://doi.org/10.1016/j.bbr.2013.08.005
Hall BJ, Slade S, Allenby C, Kutlu MG, Levin ED (2015) Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology 99:689–695. https://doi.org/10.1016/j.neuropharm.2015.03.005
Bevins RA, Besheer J, Pickett KS (2001) Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav 68(1):135–145. https://doi.org/10.1016/s0091-3057(00)00451-2
Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF (2010) Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol 21(2):153–160. https://doi.org/10.1097/FBP.0b013e328337be95
Corrigall WA, Coen KM (1991) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology 104(2):171–176. https://doi.org/10.1007/BF02244174
Neve KA, Seamans JK, Trantham-Davidson H (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24(3):165–205. https://doi.org/10.1081/rrs-200029981
Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH (1989) The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J 8(13):4025–4034
Zhang S, Xie C, Wang Q, Liu Z (2014) Interactions of CaMKII with dopamine D2 receptors: roles in levodopa-induced dyskinesia in 6-hydroxydopamine lesioned Parkinson’s rats. Sci Rep 4:6811. https://doi.org/10.1038/srep06811
Fukunaga K, Shioda N (2012) Novel dopamine D2 receptor signaling through proteins interacting with the third cytoplasmic loop. Mol Neurobiol 45(1):144–152. https://doi.org/10.1007/s12035-011-8227-8
Li CY, Mao X, Wei L (2008) Genes and (common) pathways underlying drug addiction. PLoS Comput Biol 4(1):e2. https://doi.org/10.1371/journal.pcbi.0040002
Lee AM, Messing RO (2008) Protein kinases and addiction. Ann N Y Acad Sci 1141:22–57. https://doi.org/10.1196/annals.1441.022
Fukunaga K, Miyamoto E (2000) A working model of CaM kinase II activity in hippocampal long-term potentiation and memory. Neurosci Res 38(1):3–17. https://doi.org/10.1016/s0168-0102(00)00139-5
Leon WC, Bruno MA, Allard S, Nader K, Cuello AC (2010) Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem 17(6):297–305. https://doi.org/10.1101/lm.1804410
Fan GH, Wang LZ, Qiu HC, Ma L, Pei G (1999) Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol 56(1):39–45. https://doi.org/10.1124/mol.56.1.39
Gordon N (1997) Nutrition and cognitive function. Brain Dev 19(3):165–170. https://doi.org/10.1016/s0387-7604(96)00560-8
Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T et al (2006) Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res 56(2):159–164. https://doi.org/10.1016/j.neures.2006.06.010
Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP (2003) Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res 62(3):195–204. https://doi.org/10.1016/s0920-9964(02)00284-0
Coe NR, Bernlohr DA (1998) Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim Biophys Acta 1391(3):287–306. https://doi.org/10.1016/s0005-2760(97)00205-1
Liu RZ, Li X, Godbout R (2008) A novel fatty acid-binding protein (FABP) gene resulting from tandem gene duplication in mammals: transcription in rat retina and testis. Genomics 92(6):436–445. https://doi.org/10.1016/j.ygeno.2008.08.003
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7(6):489–503. https://doi.org/10.1038/nrd2589
Owada Y, Yoshimoto T, Kondo H (1996) Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J Chem Neuroanat 12(2):113–122. https://doi.org/10.1016/s0891-0618(96)00192-5
Shioda N, Yamamoto Y, Watanabe M, Binas B, Owada Y, Fukunaga K (2010) Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J Neurosci 30(8):3146–3155. https://doi.org/10.1523/JNEUROSCI.4140-09.2010
Kawahata I, Bousset L, Melki R, Fukunaga K (2019) Fatty acid-binding protein 3 is critical for alpha-synuclein uptake and MPP(+)-induced mitochondrial dysfunction in cultured dopaminergic neurons. Int J Mol Sci 20(21). https://doi.org/10.3390/ijms20215358
Shioda N, Yabuki Y, Kobayashi Y, Onozato M, Owada Y, Fukunaga K (2014) FABP3 protein promotes alpha-synuclein oligomerization associated with 1-methyl-1,2,3,6-tetrahydropiridine-induced neurotoxicity. J Biol Chem 289(27):18957–18965. https://doi.org/10.1074/jbc.M113.527341
Cheng A, Shinoda Y, Yamamoto T, Miyachi H, Fukunaga K (2019) Development of FABP3 ligands that inhibit arachidonic acid-induced alpha-synuclein oligomerization. Brain Res 1707:190–197. https://doi.org/10.1016/j.brainres.2018.11.036
Binas B, Danneberg H, McWhir J, Mullins L, Clark AJ (1999) Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J 13(8):805–812. https://doi.org/10.1096/fasebj.13.8.805
Carboni E, Vacca C (2003) Conditioned place preference. A simple method for investigating reinforcing properties in laboratory animals. Methods Mol Med 79:481–498. https://doi.org/10.1385/1-59259-358-5:481
Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI (2013) Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology 226(4):763–768. https://doi.org/10.1007/s00213-012-2716-y
Yabuki Y, Takahata I, Matsuo K, Owada Y, Fukunaga K (2018) Ramelteon improves post-traumatic stress disorder-like behaviors exhibited by fatty acid-binding protein 3 null mice. Mol Neurobiol 55(4):3577–3591. https://doi.org/10.1007/s12035-017-0587-2
Fukunaga K, Goto S, Miyamoto E (1988) Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase II in rat brain and various tissues. J Neurochem 51(4):1070–1078. https://doi.org/10.1111/j.1471-4159.1988.tb03070.x
Yabuki Y, Fukunaga K (2013) Oral administration of glutathione improves memory deficits following transient brain ischemia by reducing brain oxidative stress. Neuroscience 250:394–407. https://doi.org/10.1016/j.neuroscience.2013.07.017
Paxinos G, Franklin KBJ (2012) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edn. Academic Press
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14(2–3):69–97. https://doi.org/10.1016/0301-0082(80)90018-0
Haber SN, Fudge JL, McFarland NR (2000) Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20(6):2369–2382. https://doi.org/10.1523/jneurosci.20-06-02369.2000
Le Foll B, Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology 178(4):481–492. https://doi.org/10.1007/s00213-004-2021-5
Vezina P, McGehee DS, Green WN (2007) Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuro-Psychopharmacol Biol Psychiatry 31(8):1625–1638. https://doi.org/10.1016/j.pnpbp.2007.08.038
Unwin N (2003) Structure and action of the nicotinic acetylcholine receptor explored by electron microscopy. FEBS Lett 555(1):91–95. https://doi.org/10.1016/s0014-5793(03)01084-6
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M et al (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23(21):7820–7829. https://doi.org/10.1523/jneurosci.23-21-07820.2003
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85(14):5274–5278. https://doi.org/10.1073/pnas.85.14.5274
Marsden CA (2006) Dopamine: the rewarding years. Br J Pharmacol 147 Suppl 1(Suppl 1):S136–S144. https://doi.org/10.1038/sj.bjp.0706473
Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105(4):849–856. https://doi.org/10.1111/j.1476-5381.1992.tb09067.x
Greengard P, Allen PB, Nairn AC (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23(3):435–447. https://doi.org/10.1016/s0896-6273(00)80798-9
Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P (2004) DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44:269–296. https://doi.org/10.1146/annurev.pharmtox.44.101802.121415
Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR (2002) Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP 32 or inhibitor 1. Biol Psychiatry 51(8):612–620. https://doi.org/10.1016/s0006-3223(01)01318-x
Heyser CJ, Fienberg AA, Greengard P, Gold LH (2000) DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res 867(1–2):122–130. https://doi.org/10.1016/s0006-8993(00)02272-1
Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3(3):175–190. https://doi.org/10.1038/nrn753
Tahara S, Fukuda K, Kodama H, Kato T, Miyoshi S, Ogawa S (2001) Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J Am Coll Cardiol 38(5):1554–1563. https://doi.org/10.1016/s0735-1097(01)01558-3
Chiamulera C, Di Chio M, Tedesco V, Cantù C, Formaggio E, Fumagalli G (2008) Nicotine-induced phosphorylation of phosphorylated cyclic AMP response element-binding protein (pCREB) in hippocampal neurons is potentiated by agrin. Neurosci Lett 442(3):234–238. https://doi.org/10.1016/j.neulet.2008.07.025
Jackson KJ, Muldoon PP, Walters C, Damaj MI (2016) Neuronal calcium/calmodulin-dependent protein kinase II mediates nicotine reward in the conditioned place preference test in mice. Behav Pharmacol 27(1):50–56. https://doi.org/10.1097/fbp.0000000000000189
Narita M, Matsumura Y, Ozaki S, Ise Y, Yajima Y, Suzuki T (2004) Role of the calcium/calmodulin-dependent protein kinase ii (CaMKII) in the morphine-induced pharmacological effects in the mouse. Neuroscience 126(2):415–421. https://doi.org/10.1016/j.neuroscience.2004.03.006
Bahk JY, Li S, Park MS, Kim MO (2002) Dopamine D1 and D2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Prog Neuro-Psychopharmacol Biol Psychiatry 26(6):1095–1104. https://doi.org/10.1016/s0278-5846(02)00243-9
Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A (2004) Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem 90(5):1094–1103. https://doi.org/10.1111/j.1471-4159.2004.02574.x
Fremeau RT Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG (1991) Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A 88(9):3772–3776. https://doi.org/10.1073/pnas.88.9.3772
Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD (2008) Significant association of DRD1 with nicotine dependence. Hum Genet 123(2):133–140. https://doi.org/10.1007/s00439-007-0453-9
Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G (2006) Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacology 184(3–4):447–455. https://doi.org/10.1007/s00213-005-0211-4
Goutier W, Lowry JP, McCreary AC, O'Connor JJ (2016) Frequency-dependent modulation of dopamine release by nicotine and dopamine D1 receptor ligands: an in vitro fast cyclic voltammetry study in rat striatum. Neurochem Res 41(5):945–950. https://doi.org/10.1007/s11064-015-1786-8
Goutier W, O'Connor JJ, Lowry JP, McCreary AC (2015) The effect of nicotine induced behavioral sensitization on dopamine D1 receptor pharmacology: an in vivo and ex vivo study in the rat. Eur Neuropsychopharmacol 25(6):933–943. https://doi.org/10.1016/j.euroneuro.2015.02.008
Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG, Smith RF (2016) Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain Struct Funct 221(1):133–145. https://doi.org/10.1007/s00429-014-0897-3
David V, Besson M, Changeux JP, Granon S, Cazala P (2006) Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 50(8):1030–1040. https://doi.org/10.1016/j.neuropharm.2006.02.003
Bahi A, Dreyer JL (2012) Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology 222(1):141–153. https://doi.org/10.1007/s00213-011-2630-8
Rosa HZ, Barcelos RCS, Segat HJ, Roversi K, Dias VT, Milanesi LH, Burger ME (2020) Physical exercise modifies behavioral and molecular parameters related to opioid addiction regardless of training time. Eur Neuropsychopharmacol 32:25–35. https://doi.org/10.1016/j.euroneuro.2019.12.111
Brené S, Lindefors N, Herrera-Marschitz M, Persson H (1990) Expression of dopamine D2 receptor and choline acetyltransferase mRNA in the dopamine deafferented rat caudate-putamen. Exp Brain Res 83(1):96–104. https://doi.org/10.1007/bf00232197
Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF et al (2010) The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem 285(47):36625–36634. https://doi.org/10.1074/jbc.M110.159954
Surmeier DJ, Song WJ, Yan Z (1996) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16(20):6579–6591. https://doi.org/10.1523/jneurosci.16-20-06579.1996
Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK (2000) Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology 152(3):343–350. https://doi.org/10.1007/s002130000548
Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK (2000) Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav 67(4):693–699. https://doi.org/10.1016/s0091-3057(00)00414-7
Ikemoto S, Qin M, Liu ZH (2006) Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci 26(3):723–730. https://doi.org/10.1523/jneurosci.4542-05.2006
Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD (2011) Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci U S A 108(10):4206–4211. https://doi.org/10.1073/pnas.1101424108
Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS et al (2004) Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42(4):653–663. https://doi.org/10.1016/s0896-6273(04)00265-x
Maura G, Giardi A, Raiteri M (1988) Release-regulating D-2 dopamine receptors are located on striatal glutamatergic nerve terminals. J Pharmacol Exp Ther 247(2):680–684
Papp M, Gruca P, Willner P (2002) Selective blockade of drug-induced place preference conditioning by ACPC, a functional NDMA-receptor antagonist. Neuropsychopharmacology 27(5):727–743. https://doi.org/10.1016/s0893-133x(02)00349-4
Wang LP, Li F, Shen X, Tsien JZ (2010) Conditional knockout of NMDA receptors in dopamine neurons prevents nicotine-conditioned place preference. PLoS One 5(1):e8616. https://doi.org/10.1371/journal.pone.0008616
Jackson A, Nesic J, Groombridge C, Clowry O, Rusted J, Duka T (2009) Differential involvement of glutamatergic mechanisms in the cognitive and subjective effects of smoking. Neuropsychopharmacology 34(2):257–265. https://doi.org/10.1038/npp.2008.50
Funding
This research was funded by the Strategic Research Program for Brain Sciences of the Japan Agency for Medical Research and Development (AMED) (grant numbers JP18dm0107071, JP19dm0107071, and 20dm0107071) to K.F. W.J. is a recipient of funding from the Otsuka Toshimi Scholarship Foundation.
Author information
Authors and Affiliations
Contributions
W.J. conducted the experiments and wrote the manuscript. G.W. provided technical assistance. A.C. performed methodology and validation. I.K. performed the validation and revised the manuscript. K.F. conceived, supervised, and coordinated the study; wrote, reviewed, and edited the manuscript; and secured funding.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All experimental procedures using mice were approved by the Committee on Animal Experiments at the Tohoku University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, W., Wilar, G., Kawahata, I. et al. Impaired Acquisition of Nicotine-Induced Conditioned Place Preference in Fatty Acid-Binding Protein 3 Null Mice. Mol Neurobiol 58, 2030–2045 (2021). https://doi.org/10.1007/s12035-020-02228-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02228-2