Abstract
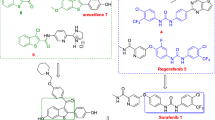
Recently, the diarylurea scaffold has been used widely to design potential anticancer agents. Pursuing our design strategy based on the modification of sorafenib as the lead compound using de novo design approaches, a new series of diaryl urea derivatives were synthesized through an efficient sequential one-pot reaction and evaluated for their in vitro antiproliferative activities against A549 and HT-29 cell lines. Notably, compound 11j exhibited antiproliferative activity against HT-29 with an IC50 value of 17.87 µM. SAR analyses revealed that substitution of the core diaryl scaffold with chlorine and methyl groups, and linear elongation of the molecules by the introduction of a methylene spacer group could cooperatively improve antiproliferative activity. The most active compound 11j induced mild apoptosis in HT-29 cells assessed based on DAPI staining experiments. The results of molecular docking simulations showed that the novel compounds bind to VEGFR-2 in a similar fashion to that observed for sorafenib. Molecular docking calculations also revealed that the most active compound 11j can bind well to the active site of VEGFR-2 by forming various interactions similar to those known for sorafenib particularly the π–π interaction, which is almost unique to sorafenib and highly active derivatives.







Similar content being viewed by others
References
Heravi MM, Mousavizadeh F, Ghobadi N, Tajbakhsh M. A green and convenient protocol for the synthesis of novel pyrazolopyranopyrimidines via a one-pot, four-component reaction in water. Tetrahedron Lett. 2014;55:1226–8. https://doi.org/10.1016/j.tetlet.2014.01.004
Weber L. Multi-component reactions and evolutionary chemistry. Drug Discov Today. 2002;7:143–7. https://doi.org/10.1016/S1359-6446(01)02090-6
Al-Bogami AS, Saleh TS, Zayed EM. Divergent reaction pathways for one-pot, three-component synthesis of novel 4H-pyrano[3,2-h]quinolines under ultrasound irradiation. Ultrason Sonochem. 2013;20:1194–202. https://doi.org/10.1016/j.ultsonch.2013.03.003
Slobbe P, Ruijter E, Orru RVA. Recent applications of multicomponent reactions in medicinal chemistry. Med Chem Comm. 2012;3:1189–218. https://doi.org/10.1039/C2MD20089A
Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. https://doi.org/10.1038/nrd2115
Wang C, Gao H, Dong J, Zhang Y, Su P, Shi Y, et al. Biphenyl derivatives incorporating urea unit as novel VEGFR-2 inhibitors: design, synthesis and biological evaluation. Bioorg Med Chem. 2014;22:277–84. https://doi.org/10.1016/j.bmc.2013.11.027
Gao G-R, Li M-Y, Lv Y-C, Cao S-F, Tong L-J, Wei L-X, et al. Design, synthesis and biological evaluation of biphenylurea derivatives as VEGFR-2 kinase inhibitors (II). Chinese. Chem Lett. 2016;27:200–4. https://doi.org/10.1016/j.cclet.2015.10.004
Zhao Y-F, Liu Z-J, Zhai X, Ge D-D, Huang Q, Gong P. Synthesis and in vitro antitumor activity of novel diaryl urea derivatives. Chinese Chem Lett. 2013;24:386–8. https://doi.org/10.1016/j.cclet.2013.02.004
Sun M, Wu X, Chen J, Cai J, Cao M, Ji M. Design, synthesis, and in vitro antitumor evaluation of novel diaryl ureas derivatives. Eur J Med Chem. 2010;45:2299–306. https://doi.org/10.1016/j.ejmech.2010.02.005
Cardot JM, Garcia Arieta A, Paixao P, Tasevska I, Davit B.Implementing the Biopharmaceutics Classification System in drug development: reconciling similarities, differences, and shared challenges in the EMA and US-FDA-recommended approaches.AAPS J. 2016;18:1039–46. https://doi.org/10.1208/s12248-016-9915-0
Chen JN, Wang XF, Li T, Wu DW, Fu XB, Zhang GJ, et al. Design, synthesis, and biological evaluation of novel quinazolinyl-diaryl urea derivatives as potential anticancer agents. Eur J Med Chem. 2016;107:12–25. https://doi.org/10.1016/j.ejmech.2015.10.045
Li GB, Ji S, Yang LL, Zhang RJ, Chen K, Zhong L, et al. LEADOPT: an automatic tool for structure-based lead optimization, and its application in structural optimizations of VEGFR2 and SYK inhibitors. Eur J Med Chem. 2015;93:523–38. https://doi.org/10.1016/j.ejmech.2015.02.019
Zarei O, Azimian F, Hamzeh-Mivehroud M, Shahbazi Mojarrad J, Hemmati S, Dastmalchi S. Design, synthesis, and biological evaluation of novel benzo[b]thiophene-diaryl urea derivatives as potential anticancer agents. Med Chem Res. 2020;29:1438–48. https://doi.org/10.1007/s00044-020-02559-8
Azimian F, Hamzeh-Mivehroud M, Mojarrad JS, Hemmati S, Dastmalchi S. Synthesis and biological evaluation of diaryl urea derivatives designed as potential anticarcinoma agents using de novo structure-based lead optimization approach. Eur J Med Chem. 2020;201:112461. https://doi.org/10.1016/j.ejmech.2020.112461
Li Y, Zhao Z, Liu Z, Su M, Wang R. AutoT&T v.2: an efficient and versatile tool for lead structure generation and optimization. J Chem Inf Model. 2016;56:435–53. https://doi.org/10.1021/acs.jcim.5b00691
Li Y, Zhao Y, Liu Z, Wang R. Automatic tailoring and transplanting: a practical method that makes virtual screening more useful. J Chem Inf Model. 2011;51:1474–91. https://doi.org/10.1021/ci200036m
Arai T, Kobayashi S, Oshimi N, Igarashi T, Sakurai T. Photoinduced large increase in the refractive index of N-2-thenoyloxyaryl-4-tert-butylphenoxyacetamide film. Bull Chem Soc Jpn. 2013;86:1079–81. https://doi.org/10.1246/bcsj.20130139
Mena-Rejon G, Caamal-Fuentes E, Cantillo-Ciau Z, Cedillo-Rivera R, Flores-Guido J, Moo-Puc R. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J Ethnopharmacol. 2009;121:462–5. https://doi.org/10.1016/j.jep.2008.11.012
Rahimi M, Safa KD, Salehi R. Co-delivery of doxorubicin and methotrexate by dendritic chitosan-g-mPEG as a magnetic nanocarrier for multi-drug delivery in combination chemotherapy. Polym Chem. 2017;8:7333–50
Allinger NL. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J Am Chem Soc. 1977;99:8127–34. https://doi.org/10.1021/ja00467a001
Dewar MJS, Thiel W. Ground states of molecules. 39. MNDO results for molecules containing hydrogen, carbon, nitrogen, and oxygen. J Am Chem Soc. 1977;99:4907–17. https://doi.org/10.1021/ja00457a005
Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267:727–48
Hammett LP. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J Am Chem Soc. 1937;59:96–103. https://doi.org/10.1021/ja01280a022
Hansch C, Leo A, Taft RW. A survey of Hammett substituent constants and resonance and field parameters. Chem Rev. 1991;91:165–95. https://doi.org/10.1021/cr00002a004
McTigue M, Murray BW, Chen JH, Deng Y-L, Solowiej J, Kania RS. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci U S A. 2012;109:18281–9. https://doi.org/10.1073/pnas.1207759109
Chen F, Fang Y, Zhao R, Le J, Zhang B, Huang R, et al. Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma. Eur J Med Chem. 2019;179:916–35. https://doi.org/10.1016/j.ejmech.2019.06.070
Bankston D, Dumas J, Natero R, Riedl B, Monahan M-K, Sibley R. A scaleable synthesis of BAY 43-9006: a potent Raf kinase inhibitor for the treatment of cancer. Org Process Res Dev. 2002;6:777–81. https://doi.org/10.1021/op020205n
Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1:55–68. https://doi.org/10.1021/cc9800071
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23. https://doi.org/10.1021/jm020017n
Egan WJ, Merz KM, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43:3867–77. https://doi.org/10.1021/jm000292e
Muegge I, Heald SL, Brittelli D. Simple selection criteria for drug-like chemical matter. J Med Chem. 2001;44:1841–6. https://doi.org/10.1021/jm015507e
Acknowledgements
The authors would like to thank the Biotechnology Research Center and the Research Office of Tabriz University of Medical Sciences for providing financial support under the Postgraduate Research Grant scheme for the PhD thesis of FA (Grant number 58079). The kind support by the Student Research Committee, Tabriz University of Medical Sciences (grant number: 62491) is also appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Azimian, F., Hamzeh-Mivehroud, M., Shahbazi Mojarrad, J. et al. Facile one-pot sequential synthesis of novel diaryl urea derivatives and evaluation of their in vitro cytotoxicity on adenocarcinoma cells. Med Chem Res 30, 672–684 (2021). https://doi.org/10.1007/s00044-020-02673-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02673-7