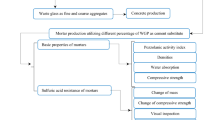
Abstract
The properties of mortars containing waste glass powder (WGP) as a cement substitute for sustainable construction at various high temperatures were investigated. For this purpose, specimens from four mixtures with WGP at various percentage levels of 0, 5, 10 and 15% were prepared and exposed to the specified temperatures. After that, the compressive and flexural strength were determined at high temperatures. The mass loss was also measured by weighing the samples before and after exposing to the high temperatures. The microstructure of mortars was analyzed by petrographic examination. Based on the obtained results, incorporation of WGP as partial replacement of cement could improve strength characteristics of the mortars at the elevated temperatures up to 17%. Also, the optimum ratio of cement replacement level was found to be 10%. In addition, the petrographic images of the mortars showed that at the same time with the strength loss of specimens, the red discoloration of WGP occurred that is attributed to the oxidation of iron compounds that starts at temperatures above 200 °C.
Similar content being viewed by others
1 Introduction
As is well known, concrete is one of the most consumed engineering material in the construction industry such as buildings, bridges and dams. High concrete consumption is due to its high strength, high resistance to fire, low cost and flexibility (Kodur 2014, Lee et al. 2018). Besides these advantages, the use of concrete also causes environmental issues that need to be taken under consideration. Manufacturing of concrete needs extensive natural resources and emits high volumes of carbon dioxide (CO2) into the atmosphere during cement production (Bui et al. 2018, Gartner and Hirao 2015).
Generally, substances such as glass that can be reused without changing or melting repeatedly, without significant alteration of its properties, have found a special place in the recycling industry (Shayan and Xu 2004, Federico and Chidiac 2009). But in some countries, the use of non-recyclable glass waste is not possible on condition that the recycling costs are expensive (Omran and Tagnit-Hamou 2016, Jin et al. 2000). The environmental problems caused by disposal of unrecycled glass in landfills are very serious (Soliman and Tagnit-Hamou 2016, Khan et al. 2019, He et al. 2019). In such cases, the researchers use the glass waste to make composite materials such as concrete (Shao et al. 2000, Idir 2009). The waste glass be utilized as aggregate or cement substitute in concrete (Aliabdo et al. 2016, Ammash et al. 2009, Tittarelli et al. 2018, Sharifi et al. 2016). The utilization of glass powder as a cement substitute could decrease cement consumption and greenhouse gas issues belonging to the production of the global cement industry (Matte et al. 2000, Ramasamy 2011). On the other hand, proper waste management is one of the most important environmental goals.
In addition to the environmental benefits, the application of waste glass powder (WGP) in concrete improves its mechanical properties and resistance to chloride ions attack (Kumarappan 2013, Khatib 2012). Tang et al. (2019) investigated the sulfate attack resistance of concrete incorporating various industrial solid wastes and reported that replacing Portland cement by solid waste materials has a positive effect on the durability of concrete exposed to sodium sulfate solution and under the same replacement ratio, WGP appears to be the most effective in offsetting the destructive effect of sulfate attack on concrete. Generally, the adoption of WGP as cement substitute can improve the strength of the concrete due to the high pozzolanic activity and filling effect of WGP grains (Patel et al. 2019, Ramdani et al. 2019). Shao et al. (Shao et al. 2000) studied the effect of adopting finely ground waste glass as cement substitute in concrete and reported that the strength of the specimen made with 30% glass powder with a 38-µm particle size was 8% higher than the control specimen. Shuhua et al. (2015) analyzed the microstructure of cement pastes containing glass powder by replacing the cement up to 40% by weight. They reported that the strength of cement pastes containing glass powder was higher than that of control mortar and glass powder can control the ASR expansion of mortars. Tamanna et al. (2016) investigated the pozzolanic reactions of glass powder in cement mortar. They reported that the pozzolanic reaction in the mortars made with glass powder causes formation of calcium silicate hydrate (C–S–H) and leads in an increase in the strength of the mortar. Patel et al. (2019) also proposed that would be effective to fill up the voids of hydrated cement matrix. Kumarappan (2013) investigated the behavior of concrete made with glass powder as cement replacement up to 40% by weight. They reported that that the optimum strength obtained by applying 10% glass powder in concrete. Also, Vandhiyan et al. (2013) studied the replacement of cement by glass powder at 5%, 10% and 15% level of replacement. They reported that glass powder improves the mechanical properties of concrete. Also, the optimum amount of glass powder was found to be 10%.
Generally, in some cases, concrete may be exposed to higher temperatures than normal conditions (e.g., fire exposure, furnaces, etc.) (Naus 2006). Although attempts have been made to evaluate the effect of heat on mortars containing glass powder, the microscopic examination of these mortars at high temperatures has not been done so far. The service life of concrete structures can be significantly shortened when concrete is exposed to high temperatures. At high temperatures, the cement paste tends to contract as a result of a moisture reduction, and on the other hand, it tends to expand due to increased temperature, while aggregates are continuously expanding due to temperature rise within the structure. These contradictory behaviors lead to cracking in the concrete. Investigating and improving the strength of concrete is important in increasing the duration of fire resistance and increasing the load capacity before and after exposure to fire. Pan et al. (2017) studied the effect of high temperatures on mortars made with glass powders as a cement substitute. They reported that mortars containing glass powder allows the pozzolanic activity and increase the strength of mortars at elevated temperatures. They also demonstrates that the CO2 emissions and energy consumption of cement paste containing glass powder were 15% and 14% less than plain mortars, respectively. Salahuddin et al. (2019) studied the effects of elevated temperature on performance of recycled coarse aggregate concrete and reported that incorporation of glass powder as binder increases the strength of recycled aggregate concrete. In another study, investigation established that application of WGP as fine aggregate improves the residual strength of self-compacting concrete at high temperatures (Ling and Poon 2014).
In this paper, the effect of WGP as a cement substitute on the mechanical and microstructure characteristics of cement pastes at elevated temperatures was studied by using petrographical methods. In addition, the mechanical properties, the mass loss performance of mortars containing WGP specimens after exposing to various high temperatures were investigated in this study. The limitations of the study were that separating the glass from other contaminated materials such as foodstuffs and chemical residues and separating the glass into its various colors precluded efficient recycling of glass into new containers. Also, large glass particles are undesirable as a component in concrete, due to a well-known alkali–silicate reaction (ASR) occurring between the silica of the glass and the alkali of other components of the concrete, which weakens the concrete (Louis P. Grasso 2008). Accordingly, it would be desirable if an efficient process for making high-quality, clean, dry powdered glass from post-consumer waste glass could be provided.
2 Experimental Details
2.1 Materials
In the present investigation, ordinary Portland cement Type II and WGP were employed for conducting studies. The chemical parameters of cement and WGP are presented in Table 1. In order to obtain WGP, group of glass cullet was crushed and heated to 710 °C. The Blain fineness of WGP was 800 m2/kg and the particle size ranged from 50 to 75 µm. Sadiqul Islam et al. (Islam et al. 2017) reported that in order to evaluate the pozzolanic effect more clearly, mortar strength tests should carried out by using superplasticizers. In addition, a polycarboxylate-based superplasticizer with 40% solid content was used in this study.
2.2 Mortar Preparation
In this study, four mortars with different levels of WGP replacement were prepared. The cement was replaced by WGP in three ratios of 5, 10 and 15% and designated as G5, G10 and G15, respectively. Similarly, another sample was made without the use of glass powder and was named G0. The mortars were prepared according to ASTM C305 (2014) and then cast into 50 × 50 × 50 mm and 40 × 40 × 160 molds for compressive and flexural strength test, respectively, and were left to set for 24 h at the room temperature. Then the specimens were demolded and cured by immersion in water before any kind of test was conducted. Table 2 indicates the mixture proportions for mortars.
2.3 Testing Procedures
2.3.1 Measurement at Elevated Temperatures
The 28 days cured specimens were allowed to dry under laboratory conditions and the mass of each specimen was measured. Also after exposure to the specified temperatures, the samples were weighed and the mass loss of each specimen was measured. At the ages of 28 days, the compressive and flexural strength tests were performed according to the ASTM C109 (2016) and ASTM C348 (2018), respectively. In order to measure the strength of the specimens at high temperatures, the specimens were first placed in an electric furnace. The heating rate of electric furnace was set to 5 °C/min to reach the specified temperature and exposed to the desired temperature for 2 h. Then the furnace is switched-off and the samples allowed to cool down for 24 h. The average of three tests was considered as the result of each experiment.
2.3.2 Microstructure Analysis
Microstructure and microscopic analysis of mortar containing WGP was performed by petrographic examination. The petrographic analysis involved production of thin sections according to the ASTM C856 (2018) standard to assist in identifying particular minerals or products of deterioration processes. For this purpose, the prepared sections of specimens containing WGP were analyzed under optical light microscope before and after exposure to high temperatures.
3 Results and Discussion
3.1 Compressive Strength
Figure 1 indicates the compressive strength results of the specimens before and after exposure to high temperature. As can be seen in this figure the compressive strength of specimens made with WGP at room temperature was higher than that of the control mortar. As for the case of 23 °C, the compressive strength of G0 was 12, 15 and 10% lower than G5, G10 and G15, respectively. The pozzolanic reactions of the glass powder particles with cement hydrates result in an increase in the strength of mortars. The pozzolanic reactions are straightly associated with fineness of the WGP.
By increasing the temperature up to 200 °C, an insignificant growth in compressive strength of all specimens was observed. This increase is primarily due to strong forces between gel particles caused by increasing temperature. Similarly, Behnood and Ziari (2008) investigated the properties of high-strength concrete after exposure to high temperatures and reported that the highest strength was recorded at the temperature of 200 °C. From 200 to 400 °C, severe strength losses occurred in all four samples, but the specimens made with WGP still had a higher compressive strength than plain mortars.
As the temperature increased up to 800 °C, the strength loss continued. At temperatures of 600 and 800 °C, the compressive strength of G0 was about 42 and 11% of its initial compressive strength at 23 °C. However, the compressive strength of G0 was lower than the other specimens at 600 °C. Meanwhile, the G10 specimen exhibited the highest compressive strength at temperatures between 23 °C and 800 °C, compared to other samples. The compressive strength of G10 at temperatures of 23, 200, 400, 600 and 800 °C were 15, 12, 11, 17 and 35% more than G0, respectively.
In accordance with observation, it was revealed that the mortars containing WGP exhibited a better compressive performance at elevated temperatures, as compared to the normal mortar. It was also found that G10 has a better thermal resistance compared to mortars containing WGP. By increasing the content of WGP more than 10% the compressive strength of the samples at various high temperatures decreases which could be due to less cement content and as a result less hydration products at 15% level of replacement of WGP in comparison with the 10% level of replacement (Pan et al. 2017).
Generally, the better fire performance of mortars containing WGP is related to the composition of more C–S–H due to the pozzolanic reaction of WGP particles. Besides, replacement of cement by WGP in the cement paste could reduce calcium hydroxide Ca(OH)2 content of the mortar. Consumption of Ca(OH)2 by the glass powder particles reactions leads to formation of stronger phases in the mortars containing WGP (Shao et al. 2000). Therefore, the samples containing WGP show superior compressive performance compared to the control mortar at elevated temperature.
3.2 Flexural Strength
Assessment and detection of the flexural behavior of mortars containing WGP at high temperatures, especially for unreinforced pavement slabs, is very important. The flexural strength results of specimens before and after exposure to high temperature are presented in Fig. 2. The flexural strength of mortars follows a similar pattern to the compressive strength of the samples from 23 to 800 °C. As can be seen, at temperatures of 23 °C, the flexural strength of the sample made with 10% WGP was higher than the other specimens. The flexural strength of G10 at the temperature of 23 °C, was about 11, 10 and 2% more than G0, G5 and G15, respectively. At 200 °C, a slight increase in the flexural strength of the samples observed. From 200 to 800 °C, the flexural strength of all samples was continuously reduced. The reduction of flexural strength was significantly increased in the temperature range of 400 to 600 °C.
According to the obtained results, it was revealed that the flexural strength of mortars made with WGP was relatively higher than plain mortars. Similar to the compressive strength, replacing the cement up to 10% improves the flexural strength of samples made with WGP at high temperatures. Also, the higher amount of WGP reduces the residual flexural strength of mortars at elevated temperatures.
In this case, the replacement percentage of cement by WGP played a noticeable role in influencing the strength losses at high temperatures. As can be seen, in the low percentage of replacement of cement with WGP, the flexural strength at various temperatures is less than other samples, while samples with a higher replacement percentage of cement with WGP exhibited higher flexural strength. This is probably due to the microfilling effect of the WGP that could improve the paste to aggregate bond in the WGP containing samples that result in greater strength of mortars in samples with higher amount of WGP. Also, the pozzolanic activity of the WGP contribute to more hydration products such as C–S–H which results in a stronger paste to aggregate bond in the mortars containing WGP (Ke et al. 2018). Therefore, the mortars containing WGP exhibited a better flexural performance than that of control mortar at elevated temperatures.
3.3 Mass Loss
Investigation of mass changes in mortars is one of the important tests in the study of behavior of mortar at high temperatures. Figure 3 presents the mass loss of with various WGP replacement ratios of 0, 5, 10 and 15% after temperature exposures at the specified temperatures of 200, 400, 600 and 800 °C.
As can be seen, all samples showed a similar pattern in mass loss. Generally, the mass changes in mortars at high temperatures are associated with physical and chemical evaporation of water in samples. The chemical evaporation of water is attributed to the rupture of hydrogen bonds in mortar. The basic strength of C–S–H at high temperatures depends on the separation of hydrogen. The hydrogen separation usually occurs at temperatures above 300 °C. The pozzolanic activity of the glass powder particles contribute to the formation of strong C–S–H bond (Vandhiyan 2013). Besides, glass powder particles can act as a filler in the paste. This causes denser microstructure in the mortars containing glass powder and consequently less mass loss. Mortars with higher densities exhibit less mass loss at high temperatures compared to the other mortars. It is probably due to the fact that the pore-water evaporation requires more energy in denser microstructures.
It can be also seen that mortars containing WGP exhibited less mass loss than that of control mortar at various temperatures. At the temperatures of 200, 400, 600 and 800 °C, the mass loss of G0 were 25, 35, 27 and 20% more than G10, respectively. Therefore, application of WGP as a cement substitute could reduce the mass loss of mortar at high temperatures. Also, lower mass loss of samples containing WGP at high temperatures indicates a stronger C–S–H bond in the mortars containing WGP.
3.4 Petrography
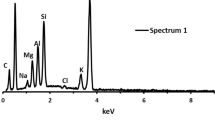
The microstructure of concrete after high temperature exposure determines its mechanical properties. For this purpose, it is important to investigate the microscopic properties of concrete after exposing to the elevated temperatures (Li and Liu 2016). Figures 4 and 6 show the petrographic images of mortars containing WGP specimens before and after being exposed to various high temperatures. Figure 4 indicates the petrographic images of G5, G10 and G15 specimens at the temperature of 23 °C. As can be seen in the petrographic images, the glass powder proved by rounded or sub-rounded shape. As can be seen in the images, there are seen a similar pattern for glass powder. The colorful appearance pattern is interpreted as WGP on petrographic observations which could be due to the variety of components in the WGP component of WGP consisting of SiO2, Na2O, CaO, MgO, Al2O3 and K2O.
The petrographic images of the mortars with various WGP replacements after the exposure to the temperature of 400 °C are presented in Fig. 5. As can be seen, by raising the temperature over 400 °C, the G5, G10 and G15 glass powder particles showed red discoloration. Also, the bottom right image shows the magnified WGP particle under the petrological microscope in thin section. Generally, the red discoloration commences at around at 300 °C, but become more visible at 400 °C. The red discoloration of glass powder is attributed to the oxidation of iron compounds that starts at 300 °C.
The petrography images of the G5, G10 and G15 after exposure to 600 °C are presented in Fig. 6. By increasing the temperature up to 600 °C, the color of glass powder particles changed to black due to heating. This could be an indication of the maximum reached temperature in the heating process of mortars containing WGP. This discoloration of WGP particles starts at around 300 °C and at the same time severe strength reduction occurs. In addition, as could be observed in Fig. 6, rising temperatures result in the crack growth in the aggregate and causes reduction in strength.
4 Conclusion
The application of waste glass powder (WGP) as a cement substitute in mortars exposed to elevated temperatures was proposed in this study. Experiments were conducted to study the mechanical and microstructural properties of these mortars. Based on the obtained results, the following conclusions can be drawn:
-
1.
Incorporation of WGP as partial replacement of cement could improve the strength of mortars at elevated temperatures. The compressive strengths of G10 at temperatures of 23, 200, 400, 600 and 800 °C were 15, 12, 11, 17 and 35% more than G0, respectively.
-
2.
Utilizing WGP in the cement mortars increases its flexural strength at all temperatures. Replacement of cement by WGP in the proportion of 10% at the specified temperatures of 23 to 800 °C increased the flexural strength of the mortars containing WGP by 11, 12, 19, 40 and 47%.
-
3.
Based on the mass loss results, mortars containing WGP exhibited less mass loss than that of control mortar at various high temperatures. This is due to the filling role of the WGP, which results in a higher density of the samples containing the WGP.
-
4.
The petrographic analysis indicated that the WGP proved by rounded shape. It is also seen that as a result of heating the glass powder showed red discoloration at 300 °C and then the color of glass powder particles changed to black at around 600 °C.
-
5.
The petrographic images of the mortars containing WGP indicated that the red discoloration of glass powder is attributed to the oxidation of iron compounds and coincides with the severe strength loss of specimens at elevated temperatures.
-
6.
The optimum percentage of replacement of cement with WGP at ambient and high temperatures was found to be 10%.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aliabdo, A. A., Abd Elmoaty, M., & Aboshama, A. Y. (2016). Utilization of waste glass powder in the production of cement and concrete. Constr Building Mater, 15(124), 866–877.
Ammash, Haider K., Muhammed, Muhammed S., & Nahhab, Ali H. (2009). Using of waste glass as fine aggregate in concrete. Al-Qadisiya J Eng Sci, 2, 206–214.
Behnood, A., & Ziari, H. (2008). Effects of silica fume addition and water to cement ratio on the properties of high-strength concrete after exposure to high temperatures. Cement Concrete Composites, 30, 106–112.
Bui, N. K., Satomi, T., & Takahashi, H. (2018). Mechanical properties of concrete containing 100% treated coarse recycled concrete aggregate. Constru Build Mater, 163, 496–507.
C109, A. 2016. Standard test method for compressive strength of hydraulic cement mortars (using 2-in. or [50-mm] cube specimens). West Conshohocken
C305, A. 2014. Standard practice for mechanical mixing of hydraulic cement pastes and mortars of plastic consistency. West Conshohocken
C348, A. 2018. Standard test method for flexural strength of hydraulic-cement mortars. West Conshohocken
C856, A. 2018. Standard practice for petrographic examination of hardened concrete. West Conshohocken, PA
Federico, L. M., & Chidiac, S. E. (2009). Waste glass as a supplementary cementitious material in concrete—Critical review of treatment methods. Cement Concrete Composites, 31, 606–610.
Gartner, E., & Hirao, H. (2015). A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cement Concrete Res, 78, 126–142.
Gopalakrishnan, R., & Govindarajan, D. (2011). Compressive strength and electron paramagnetic resonance studies on waste glass admixtured cement. New J Glass Ceramic, 1(3), 119.
He, Z.-H., Zhan, P.-M., Du, S.-G., Liu, B.-J., & Yuan, W.-B. (2019). Creep behavior of concrete containing glass powder. Composites Part B Eng, 166, 13–20.
Idir R, Cyr M, Tagnit-Hamou A. 2009. Use of waste glass as powder and aggregate in cement-based materials. 1st International Conference on Sustainable Built Environment Infrastructures in Developing Countries. Algeria.
Islam, G. M. S., Rahman, M. H., & Kazi, N. (2017). Waste glass powder as partial replacement of cement for sustainable concrete practice. Int J Sustainable Built Environ, 6, 37–44.
Jin, W., Meyer, C., & Baxter, S. (2000). Concrete with glass aggregate. ACI Mater J, 97, 208–213.
Ke, G., Li, W., Li, R., Li, Y., & Wang, G. (2018). Mitigation effect of waste glass powders on alkali–silica reaction (ASR) expansion in cementitious composite. Int J Concrete Struct Mater, 12, 67.
Khan, Q. S., Sheikh, M. N., Mccarthy, T. J., Robati, M., & Allen, M. (2019). Experimental investigation on foam concrete without and with recycled glass powder: a sustainable solution for future construction. Constru Building Mater, 201, 369–379.
Khatib, J. M., Negim, E. M., Sohl, H. S., & Chileshe, N. (2012). Glass powder utilisation in concrete production. Eur J Appl Sci, 4(4), 173–176.
Kodur, V. (2014). Properties of concrete at elevated temperatures. ISRN Civil Eng, 2014, 15.
kumarappan, N. (2013). Partial replacement cement in concrete using waste glass. Int J Eng Res Technol, 2, 1880–1883.
Lee, H., Hanif, A., Usman, M., Sim, J., & Oh, H. (2018). Performance evaluation of concrete incorporating glass powder and glass sludge wastes as supplementary cementing material. J Cleaner Prod, 170, 683–693.
Li, H., & Liu, G. (2016). Tensile properties of hybrid fiber-reinforced reactive powder concrete after exposure to elevated temperatures. Int J Concrete Struct Mater, 10, 29–37.
Ling, T.-C., & Poon, C.-S. (2014). High temperatures properties of barite concrete with cathode ray tube funnel glass. Fire Mater, 38, 279–289.
Liu, S., Xie, G., & Wang, S. (2015). Effect of glass powder on microstructure of cement pastes. Adv Cement Res, 27(5), 259–267.
Louis P. Grasso, J. L. P. G., Sr. patrick S. Grasso, Sr. elliot krackocynthia A. Andelaralph J. Acampora. 2008. Production of glass powder from waste glass, and products made using the same, especially concrete. US patent application.
Matte, V., Moranville, M., Adenot, F., Richet, C., & Torrenti, J. M. (2000). Simulated microstructure and transport properties of ultra-high performance cement-based materials. Cement Concrete Res, 30, 1947–1954.
Naus DJ. 2006. The effect of elevated temperature on concrete materials and structures –a literature review. United States.
Omran, A., & Tagnit-hamou, A. (2016). Performance of glass-powder concrete in field applications. Construct Build Mater, 109, 84–95.
Pan, Z., Tao, Z., Murphy, T., & Wuhrer, R. (2017). High temperature performance of mortars containing fine glass powders. J Cleaner Prod, 162, 16–26.
Patel, D., Tiwari, R. P., Shrivastava, R., & Yadav, R. K. (2019). Effective utilization of waste glass powder as the substitution of cement in making paste and mortar. Const Build Mater, 199, 406–415.
Ramdani, S., Guettala, A., Benmalek, M. L., & Aguiar, J. B. (2019). Physical and mechanical performance of concrete made with waste rubber aggregate, glass powder and silica sand powder. J Build Eng, 21, 302–311.
Salahuddin, H., Nawaz, A., Maqsoom, A., Mehmood, T., & Zeeshan, B. U. A. (2019). Effects of elevated temperature on performance of recycled coarse aggregate concrete. Constr Build Mater, 202, 415–425.
Shao, Y., Lefort, T., Moras, S., & Rodriguez, D. (2000). Studies on concrete containing ground waste glass. Cement Concrete Res, 30, 91–100.
Sharifi, Y., Afshoon, I., Firoozjaei, Z., & Momeni, A. (2016). Utilization of waste glass micro-particles in producing self-consolidating concrete mixtures. Int J Concrete Struct Mater, 10, 337–353.
Shayan, A., & Xu, A. (2004). Value-added utilisation of waste glass in concrete. Cement Concrete Res, 34, 81–89.
Soliman, N. A., & Tagnit-hamou, A. (2016). Development of ultra-high-performance concrete using glass powder—Towards ecofriendly concrete. Constru Build Mater, 125, 600–612.
Tamanna, N., Sutan, N., Tuladhar, Rabin, Lee, D., & Yakub, I. (2016). Pozzolanic properties of glass powder in cement paste. J Civil Eng Sci Technol, 7, 75–81.
Tang, Z., Li, W., Ke, G., Zhou, J. L., & Tam, V. W. Y. (2019). Sulfate attack resistance of sustainable concrete incorporating various industrial solid wastes. J Cleaner Prod, 218, 810–822.
Tittarelli, F., Giosue, C., & Mobili, A. (2018). Recycled glass as aggregate for architectural mortars. Int J Concrete Struct Mater, 12, 57.
Vandhiyan, R., Ramkumar, K., & Ramya, R. (2013). Experimental study on replacement of cement by glass powder. Int J Eng Res Technol, 2, 234–238.
Acknowledgements
The authors would like to appreciate Isfahan University of Technology for its financial support.
Author’s information
AS, HM, MB and MV are M. Sc. Graduates at the Isfahan University of Technology. KB is an Associate Professor at the Isfahan University of Technology.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AS, KB, and MB analyzed the test results and drafted the manuscript, HM, MB, and MV carried out the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Journal information: ISSN 1976-0485 / eISSN 2234-1315
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sedaghatdoost, A., Behfarnia, K., Moosaei, H. et al. Investigation on the Mechanical Properties and Microstructure of Eco-friendly Mortar Containing WGP at Elevated Temperature. Int J Concr Struct Mater 15, 1 (2021). https://doi.org/10.1186/s40069-020-00434-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40069-020-00434-9