Abstract
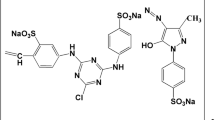
A seaweed Ulva reticulata was used to synthesis biochar, and it was used in the removal of Reactive Red 120 (R120). The optimum thermal pyrolysis temperature was found to be 300 °C, and it was confirmed by proximate and elemental analysis of the biochar. The practical applicability of this biochar was explored by conducting the experiments in batch and continuous mode. An up-flow packed bed reactor was used to study the removal of reactive red 120 in continuous mode. The operating parameters like solution pH, contact time, biochar dosage, temperature, initial concentration were studied. A four-parameter model Fritz-Schlunder-IV and pseudo-first-order kinetic model best fitted the experimental uptake with a correlation coefficient of 0.9996 and 0.9951, respectively. The maximum removal efficiency and uptake capacity of 84.2% and 210.5 mg/g were obtained at operating conditions of pH of 2, biochar dosage of 2 g/L, temperature of 30 °C, and initial concentration of 500 mg/L. The partition coefficient was studied to overcome the limitation of the adsorption capacity, and the highest partition coefficient was obtained as 4.13 L/g at 100% breakthrough time with a removal efficiency and uptake capacity of 89.2% and 111.5 mg/g. The thermodynamic study concluded that reactions are spontaneous and endothermic. The continuous study concluded that the uptake capacity of 100.71 mg/g was obtained at operating conditions of bed depth of 25 cm, the flow rate of 0.48 L/h, and initial dye concentration of 250 mg/L.











Similar content being viewed by others
References
Abdolali A, Guo WS, Ngo HH et al (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour Technol 160(2014):57–66. https://doi.org/10.1016/j.biortech.2013.12.037
Felista MM, Wanyonyi WC, Ongera G (2020) Adsorption of anionic dye (Reactive black 5) using macadamia seed Husks: kinetics and equilibrium studies. Sci African. https://doi.org/10.1016/j.sciaf.2020.e00283
Gokulan R, Avinash A, Prabhu GG, Jegan J (2019) Remediation of remazol dyes by biochar derived from Caulerpa scalpelliformis - an eco-friendly approach. J Environ Chem Eng 7(5):103297. https://doi.org/10.1016/j.jece.2019.103297
Miladinova PM, Vaseva RK, Lukanova VR (2015) Synthesis and investigation of some acid azo dyes for wool. J Chem Technol Metall 22(2000):49–54
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interf Sci 209(2014):172–184. https://doi.org/10.1016/j.cis.2014.04.002
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination. 280(2011):1–13. https://doi.org/10.1016/j.desal.2011.07.019
Fegousse A, El Gaidoumi A, Miyah Y et al (2019) Pineapple bark performance in dyes adsorption: optimization by the central composite design. J Chem. https://doi.org/10.1155/2019/3017163
Franca AS, Oliveira LS, Ferreira ME (2009) Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination. https://doi.org/10.1016/j.desal.2008.11.017
Han R, Zhang L, Song C et al (2010) Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym 79(4):1140–1149. https://doi.org/10.1016/j.carbpol.2009.10.054
Lehmann J, Joseph S (2012) Biochar for environmental management: an introduction. In: Biochar for Environmental Management: Science and Technology
Liu Y, Liu YJ (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61(3):229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL et al (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159(12):3269–3282. https://doi.org/10.1016/j.envpol.2011.07.023
Rameshkumar S, Ramakritinan C., Yokeshbabu M (2012) Proximate composition of some selected seaweeds from Palk bay and Gulf of Mannar, Tamilnadu, India. Asian J Biomed Pharm Sci
Cui Y, Kang W, Qin L et al (2020) Magnetic surface molecularly imprinted polymer for selective adsorption of quinoline from coking wastewater. Chem Eng J. https://doi.org/10.1016/j.cej.2020.125480
Gokulan R, Ganesh Prabhu G, Avinash A, Jegan J (2020) Experimental and chemometric analysis of bioremediation of remazol dyes using biochar derived from green seaweeds. Desalin Water Treat 184:340–353. https://doi.org/10.5004/dwt.2020.25339
Paul J, Rawat KP, Sarma KSS, Sabharwal S (2011) Decoloration and degradation of Reactive Red-120 dye by electron beam irradiation in aqueous solution. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2011.03.009
Abdin Y, Usman A, Ok YS et al (2020) Competitive sorption and availability of coexisting heavy metals in mining-contaminated soil: contrasting effects of mesquite and fishbone biochars. Environ Res. https://doi.org/10.1016/j.envres.2019.108846
Shahzad A, Jang J, Lim SR, Lee DS (2020) Unique selectivity and rapid uptake of molybdenum-disulfide-functionalized MXene nanocomposite for mercury adsorption. Environ Res. https://doi.org/10.1016/j.envres.2019.109005
Sivarajasekar N, Baskar R (2014) Adsorption of basic red 9 onto activated carbon derived from immature cotton seeds: isotherm studies and error analysis. Desalin Water Treat. https://doi.org/10.1080/19443994.2013.834518
Gokulan R, Ganesh Prabhu G, Jegan J (2019) A novel sorbent Ulva lactuca-derived biochar for remediation of Remazol Brilliant Orange 3R in packed column. Water Environ Res 91(7):642–649. https://doi.org/10.1002/wer.1092
Kim WK, Shim T, Kim YS et al (2013) Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour Technol 138:266–270. https://doi.org/10.1016/j.biortech.2013.03.186
Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287. https://doi.org/10.1111/j.1438-8677.1999.tb00253.x
Armstrong J, Armstrong W (1991) A convective through-flow of gases in Phragmites australis (Cav.) Trin. ex Steud. Aquat Bot 39:75–88. https://doi.org/10.1016/0304-3770(91)90023-X
Mupa M, Rutsito DD, Musekiwa C (2016) Removal of methylene blue from aqueous solutions using biochar prepared from Eichhorrnia crassipes (water hyacinth)-molasses composite: kinetic and equilibrium studies. Afr J Pure Appl Chem 10(6):63–72. https://doi.org/10.5897/ajpac2016.0703
Jeguirim M, Limousy L, Dutournie P (2014) Pyrolysis kinetics and physicochemical properties of agropellets produced from spent ground coffee blended with conventional biomass. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2014.04.018
Aksu Z, Çaǧatay ŞŞ (2006) Investigation of biosorption of Gemazol Turquise Blue-G reactive dye by dried Rhizopus arrhizus in batch and continuous systems. Sep Purif Technol 48:24–35. https://doi.org/10.1016/j.seppur.2005.07.017
Tangaromsuk J, Pokethitiyook P, Kruatrachue M, Upatham ES (2002) Cadmium biosorption by Sphingomonas paucimobilis biomass. Bioresour Technol 85:103–105. https://doi.org/10.1016/S0960-8524(02)00066-4
Vijayaraghavan K, Yun YS (2008) Competition of Reactive red 4, Reactive orange 16 and Basic blue 3 during biosorption of Reactive blue 4 by polysulfone-immobilized Corynebacterium glutamicum. J Hazard Mater 153(1):478–486. https://doi.org/10.1016/j.jhazmat.2007.08.079
Gerçel Ö, Gerçel HF (2007) Adsorption of lead(II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem Eng J. https://doi.org/10.1016/j.cej.2007.01.010
Padmesh TVN, Vijayaraghavan K, Sekaran G, Velan M (2006) Application of two- and three-parameter isotherm models: biosorption of acid red 88 onto Azolla microphylla. Bioremediat J 10(1–2):37–44. https://doi.org/10.1080/10889860600842746
Vikrant K, Kim KH (2019) Nanomaterials for the adsorptive treatment of Hg (II) ions from water. Chem. Eng. J
Ravindiran G, Jeyaraju RM, Josephraj J, Alagumalai A (2019) Comparative desorption studies on remediation of remazol dyes using biochar (sorbent) derived from green marine seaweeds. ChemistrySelect. 4(25):7437–7445. https://doi.org/10.1002/slct.201901348
Lu MC, Biel LCC, Wan MW et al (2016) Adsorption of dibenzothiophene sulfone from fuel using chitosan-coated bentonite (CCB) as biosorbent. Desalin Water Treat 57(11):5108–5118. https://doi.org/10.1080/19443994.2014.996773
Ong HR, Khan MR, Yousuf A et al (2015) Effect of waste rubber powder as filler for plywood application. Pol J Chem Technol 17(1):41–47. https://doi.org/10.1515/pjct-2015-0007
Vijayaraghavan K, Thilakavathi M, Palanivelu K, Velan M (2005) Continuous sorption of copper and cobalt by crab shell particles in a packed column. Environ Technol 26:267–276. https://doi.org/10.1080/09593332608618566
Smebye AB, Sparrevik M, Schmidt HP, Cornelissen G (2017) Life-cycle assessment of biochar production systems in tropical rural areas: comparing flame curtain kilns to other production methods. Biomass Bioenergy 101:35–43. https://doi.org/10.1016/j.biombioe.2017.04.001
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836
Silva JP, Canchala TR, Lubberding HJ, Peña EJ, Gijzen HJ (2016) Greenhouse gas emissions from a tropical eutrophic freshwater wetland. Int J Environ Ecol Eng 10(5):541–547. https://doi.org/10.5281/zenodo.1124217
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, M., Gokulan, R., Sujatha, S. et al. Biodecolorization of Reactive Red 120 in batch and packed bed column using biochar derived from Ulva reticulata. Biomass Conv. Bioref. 13, 1707–1721 (2023). https://doi.org/10.1007/s13399-020-01268-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01268-x