Abstract
Streptococcus agalactiae (S. agalactiae) is an important pathogen that can lead to neonatus and mother infection. The current existing techniques for the identification of S. agalactiae are limited by accuracy, speed and high-cost. Therefore, a new multiple cross displacement amplification (MCDA) assay was developed for test of the target pathogen immediately from vaginal and rectal swabs. MCDA primers screening were conducted targeting S. agalactiae pcsB gene, and one set of MCDA primers with better rapidity and efficiency was selected for establishing the S. agalactiae-MCDA assay. As a result, the MCDA method could be completed at a constant temperature of 61 °C, without the requirement of special equipment. The detection limit is 250 fg (31.5 copies) per reaction, all S. agalactiae strains displayed positive results, but not for non-S. agalactiae strains. The visual MCDA assay detected 16 positive samples from 200 clinical specimen, which were also detected positive by enrichment/qPCR. While the CHROMagar culture detected 6 positive samples. Thus, the MCDA assay is prefer to enrichment/qPCR and culture for detecting S. agalactiae from clinical specimen. Particularly, the whole test of MCDA takes about 63.1 min, including sample collection (3 min), DNA preparation (15 min), MCDA reaction (45 min) and result reporting (6 s). In addition, the cost was very economic, with only US$ 4.9. These results indicated that our S. agalaciae-MCDA assay is a rapid, sensitive and cost-efficient technique for target pathogen detection, and is more suitable than conventional assays for an urgent detection, especially for 'on-site' laboratories and resource-constrained settings.
Similar content being viewed by others
Introduction
Streptococcus agalactiae is a gram-positive group B Streptococcus (GBS) that is associated with the asymptomatic colonization of human urogenital and gastrointestinal tracts (Kwatra et al. 2016; Vieira et al. 2019). However, this opportunistic pathogen can cause serious infections in susceptible hosts, especially in neonates, leading to pneumonia, sepsis and meningitis (Boyer et al. 1985; McGee et al. 2010; Rosa-Fraile et al. 2017). As S. agalactiae could be vertically transmitted from a colonized mother to her babies, prenatal screening was recommended for those gestational age from 35 to 37 weeks or those who in faced with the risk of premature labor in industrial countries (Verani et al. 2010; Rabaan et al. 2017). When the positive results were reported, those high-risk pregnant women will accept antibiotic treatment to prevent the occurrence of vertical transmission (Curry et al. 2018). Owing to these prevention strategies, the incidence of neonatal diseases attributing to S. agalactiae significantly reduced in western countries (Verani et al. 2010). However, pregnant women who screened positive and received antibiotic treatment whose babies subsequently still infected with S. agalactiae. This may be due to the fact that S. agalactiae colonization was intermittent (Hansen et al. 2004; Feuerschuette et al. 2018). When antenatal screening conducted more than a certain time before delivery could not effectively predict the risk of infection (Yancey et al. 1996). Therefore, rapid screening techniques at the time of delivery were needed to do to prevent the occurrence of serious early-onset disease.
Currently, the gold standard used for the detection and identification of S. agalactiae was the enrichment culture, followed by biochemical analyses (Verani et al. 2010; Tickler et al. 2019). As the enrichment culture usually takes 48–72 h for obtaining the final result, which is a too long time for those women at delivery (Venkatesh et al. 2010; Connell et al. 2007; Kim et al. 2015). Moreover, the enrichment culture is with low sensitivity, leading to some false-negative results (Lin et al. 2019). Thus, a more sensitive method is required to complement the current method. Then, many rapid and sensitive molecular methods have been developed for S. agalactiae, and 4 commercially available GBS molecular diagnostic tests based on qPCR were applied in detecting S. agalactiae from Lim Broth culture about 18–24 h of vaginal-rectal swab specimens, including the Panther Fusion GBS assay, the BD MAXTM GBS assay, the ARIESGBS assay and the Xpert GBS LB assay (Shin et al. 2019; Vieira et al. 2019). Although these qPCR reduced the turn-around time and increased the sensitivity, it depends on a specialised laboratory and well-trained personnel, which limited its apply in primary-level medical and health care institutions. The other loop-mediated isothermal DNA amplification techniques were also developed for detecting S. agalactiae in clinical specimens, and the results were usually evaluated by the real time turbidimeter (a GENIE II fluorometer). However, the cost of this device was about £ 5000, which is still a burden in resource-limited hospitals in developing countries. Thus, a more rapid, free of special equipment and cost test of S. agalactiae directly from patient specimens is in an urgent need to satisfy the clinical requirement.
A new multiple cross displacement amplification technique was developed in recent years (Wang et al. 2017). This method avoid the long turn-around-time that required for enrichment culture, and the complex devices which is required for qPCR. It could be completed at a constant temperature in 45 min. A set of ten primers were designed in the MCDA assay, including displacement primers F1 and F2, cross primers CP1 and CP2, and amplification primers C1, C2, D1, D2, R1 and R2. These primers anneal to the target gene, and the polymerase extends in tandem yielding different-sized products, including CP1/D1 products, CP1/C1 products, R1/R1s products, and so on (Wang et al. 2017). Compared to the six primers of the LAMP assay, the MCDA assay of ten primers in theory is more specific. Moreover, according to the published articles, the LAMP assay is not as sensitive as the MCDA assay (Zhao et al. 2019a, b). Here, the judgment of the result was obtained by naked eyes without opening the lid, and thus preventing the aerosol pollution. All these merits made it a promise tool for point-of-care test for pathogen.
In this study, we developed a visual MCDA assay for S. agalactiae targeting pcsB gene, which can achieve rapid, sensitive and specific detection of the target pathogen. To further confirm the clinical application value of the MCDA assay, we collected 200 vaginal and rectal swabs from pregnant women, and compared it to the detecting result of the enrichment/qPCR assay and culture.
Materials and methods
Reagents
We purchased TIANamp Bacteria DNA Kits from Tiangen Biotech Co., Ltd.(Beijing, China). Lysozyme and agarose were purchased from Beyotime Biotechnology Co., Ltd.(Shanghai, China). The isothermal amplification kits and Malachite Green were purchased from Huidexin Bio-technology Co., Ltd.(Tianjin, China). qPCR Mix, pMD19-T Vector and DH5α were purchased from Takara Biomedical Technology Co., Ltd. (Beijing, China). The The swabs were purchased from Copan Diagnostics, Inc. (Lombardy, Italy). Todd Hewitt medium, gentamicin and nalidixic were obtained from Qingdao Hi-Tech Industrial Park Haibo Biotechnology Co., Ltd(Qingdao, China).
Genomic DNA extraction
The analytical specificity of the MCDA assay was evaluated by 28 strains (Table 1), including 7 S. agalactiae strains and 21 non-S. agalactiae strains. S. agalactiae strain ATCC12386 was employed as a standard strain in this study. We collected these strains from the Department of microbial Laboratory, Taihe Hospital of Hubei University of Medicine, and identified these strains by DL-96 systems using 96E ID Card and conventional PCR targeting 16s rDNA and sequencing. Genomic DNA were extracted using a DNA Mini Kit following the manufacturers instructions, and extracted DNA was stored at – 80 °C until use. The purity and concentration of these extracted genomic DNA was determined using ultraviolet spectrophotometer (Nano drop one, Thermo, Massachusetts, USA) at A260/280.
Primer design
The pcsB gene encodes a putative peptidoglycan hydrolase, which is necessary for cell wall. Here, the software PRIMER Premier 5.0 and PrimerExplorer V4 (http://primerexplorer.jp/elamp4.0.0/index.html) were used to design MCDA primers targeting pcsB gene of S. agalactiae. The MCDA primers were demonstrated in Table 2 and Additional file 1: Table S1, and the schematic diagram was showed in Fig. 1. All MCDA primers were synthesized and purified by TsingKe Biotech Co., Ltd. (Beijing, China) at HPLC purification grade.
MCDA reactions
The pcsB-MCDA reaction reagents were prepared as follows: 0.4 µM displacement primers F1 and F2, 0.8 µM amplification primers C1, C2, D1, D2, R1 and R2, 1.6 µM cross primer CP1 and CP2, 1 µL Bst DNA polymerase (10U), 12.5 µL 2*Reaction Buffer, 1.5 µL Malachite Green and 1 µL DNA template.
The pcsB-MCDA products were analyzed by Malachite Green (MG), and further confirmed by gel electrophoresis. MG caused the color of reaction solution turning from green to bright green at the end of amplification for S. agalactiae, but turning colorless for non-S. agalactiae. A ladder-like band appeared at 2% gel electrophoresis for S. agalactiae, but no ladder-like bands were observed for non-S. agalactiae. Six different temperatures (ranging from 60 °C to 65 °C at 1 °C interval) were selected to evaluate the optimum amplification temperature.
Sensitivity and specificity of the MCDA assay
Under the optimum condition, a tenfold serial dilution (from 2.5 ng to 2.5 fg) of S. agalactiae templates (ATCC12386) or the pMD19-T plasmid (from 3.15 * 106 copies to 3.15 copies) was prepared for testing the sensitivity of the MCDA assay. The pMD19-T plasmid contained the target gene of pcs B, which was constructed according to the vector construction protocol. The specificity of the S. agalactiae-MCDA assay was evaluated using genomic DNA extracted from S. agalactiae and non-S. agalactiae strains.
S. agalactiae culture
The vaginal and rectal swabs were inoculated on CHROMagar plates, and incubated at 37 °C for 24 h. The suspicious purple colonies were identified by the CAMP test, and further determined by conventional PCR and sequencing. The primer sequences were 8F(5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R(5′-GGTTACCTTGTTACGACTT-3′) (Turner et al. 1999).
S. agalactiae-MCDA analysis of vaginal and rectal swabs
In order to further confirm the practicability of the S. agalactiae-MCDA assay, we directly collected 200 vaginal and rectal swabs from pregnant women with gestational age ≥ 24 weeks. Those pregnant women who used antibiotics in the 30 days prior to specimen collection were excluded. The swabs were transported at Copan’s medium. Each swab head circled about 10 times in 80 µL DW, and 20 µL were used for CHROMagar culture. We also added the other 20 µL to Todd Hewitt selective medium for enrichment culture about 24 h, and the DNA was extracted as ever reported (Vieira et al. 2019). The remaining solution were boiled at 100 °C for 15 min. When the temperature of the solution declined to room temperature, 2 µL DNA were directly used for MCDA detection. The qPCR primer sequences were 5′-TTTCACCAGCTGTATTAGAAGTA-3′ and 5′-GTTCCCTGAACATTATCTTTGAT-3′ (Vieira et al. 2019). We conducted qPCR reactions in a 20 µL volume that contained 0.4 µM forward and reverse primer each, 10 µL TB Green Fast qPCR Mix, and 2.0 µL DNA. The amplification was conducted at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 34 s.
Results
Primers screening
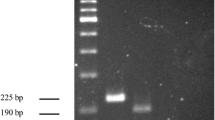
In order to achieve the optimum amplification situation of S. agalactiae, three sets of MCDA primers targeting pcsB gene were designed. All these primers were conducted in MCDA reaction. 2% agarose gel electrophoresis was used to analyze the MCDA products of these three sets of MCDA primers. As observed in Fig. 2, the results showed that the ladder bands for the second set of MCDA primers were the brightest. Thus, the second set of MCDA primers was employed for the following experiments.
Confirmation of MCDA products
Three groups of genomic DNA were prepared to confirm the feasibility of the secreeded MCDA primers, including the first group of S. agalactiae ATCC12386, the second group of S. pyogenes, and the third group of S. pneumoniae. These DNA were respectively added to the MCDA mixture, and incubated at 62 °C for 1 h. At the end of amplification, MG and gel electrophoresis were employed to analyze MCDA products. By MG, light green were visual for S. agalactiae, and colorless were visual for S. pyogenes, S. pneumoniae and DW (Fig. 3). A ladder-like bands appeared at 2% gel electrophoresis for S. agalactiae, but no ladder-like bands were observed for negative control of S. pyogenes, S. pneumoniae and blank control of DW (Fig. 3). These results demonstrated that the second set of MCDA primers designed here could be applied in detecting S. agalactiae.
Confirmation of S. agalactiae-MCDA products. Methods of Malachite Green (a) and 2% Agarose gel electrophoresis (b) were used to analyze MCDA products. Lane M, DNA maker DL1000. Tube1/Lane1, positive group (S. agalactiae strain ATCC12386); Tube2/Lane2, negative group (Streptococcus pyogenes); Tube3/Lane3, negative group (S. pneumoniae); Tube4/Lane4, blank group (DW)
The optimum amplification temperature of the S. agalactiae-MCDA assay
The optimum temperature screening of the S. agalactiae-MCDA reaction were conducted at six different temperatures varing from 60 to 65 °C with 1 °C intervals for 45 min. As observed in Fig. 4, the color of tube 1, tube 2, tube 3 and tube 4 were almost the same, and were more darker than that of tube 5 and tube 6. By 2% agarose gel electrophoresis, the brightest ladder bands were observed at 61 °C. Thus, the subsequent experiments were completed at 61 °C.
Temperature screening. 2.5 pg S. agalactiae strain ATCC12386 genome in MCDA reaction were respectively amplified at six different temperatures varing from 60 to 65 °C with 1 °C intervals. Then, we evaluate the amplification efficiency of the S. agalactiae-MCDA assay by using two methods, including Malachite Green and Agarose gel electrophoresis
Sensitivity of the S. agalactiae-MCDA assay
Sensitivity of the MCDA assay was analyzed using S. agalactiae ATCC12386 genome and the pMD19-T plasmid. The genome were diluted from 2.5 ng µL−1 to 2.5 fg µL−1. The plasmid were diluted from 3.15 * 106 copies µL−1 to 3.15 copies µL−1. Then, the different concentration of DNA was added to the MCDA reaction, which was incubated at 61 °C for 1 h. As observed in Fig. 5, the results showed that the detection limit of the MCDA assay was 250 fg µL−1 (31.5 copies µL−1), which was in accordance with that of the gel electrophoresis detection.
Sensitivity analysis. Tenfold serial dilutions of the template (2.5 ng, 250 pg, 25 pg, 2.5 pg, 250 fg, 25 fg, 2.5 fg) or the pMD19-T plasmid(3.15 * 106 copies, 3.15 * 105 copies, 3.15 * 104 copies, 3.15 * 103 copies, 3.15 * 102 copies, 3.15 * 10 copies, 3.15 copies) were added to standard MCDA reactions and incubated at 61 °C for 1 h, respectively. Diagnosis techniques, including colorimetric indicator (a, c) and gel electrophoresis (b,d) were used for analysis of MCDA amplicons. Tubes (a)/Lanes (b) 1–8 respectively represent S. agalactiae strain ATCC12386 DNA levels of 2.5 ng, 250 pg, 25 pg, 2.5 pg, 250 fg, 25 fg, 2.5 fg per reaction, and a blank control (DW). Tubes (c)/Lanes (d) 1–8 respectively represent the pMD19-T plasmid levels of 3.15 * 106 copies, 3.15 * 105 copies, 3.15 * 104 copies, 3.15 * 103 copies, 3.15 * 102 copies, 3.15 * 10 copies, 3.15 copies per reaction, and a blank control (DW)
Specificity of the S. agalactiae-MCDA assay
DNA extracted from 7 S. agalactiae strains and 21 non-S. agalactiae strains were used to evaluate the specificity performance of the MCDA assay. As observed in Fig. 6, all these S. agalactiae strains showed positive results, where light green was visual. However, colourless was visual for non-S. agalactiae strains.
Specificity analysis. A total of 28 strains were used to evaluate the specificity of MCDA-LFB assays. Light green was visuable for all Streptococcus agalactiae, and colorless was observed for non-Streptococcus agalactiae. 1, Positive control (Streptococcus agalactiae strain ATCC12386), 2–7, Streptococcus agalactiae. 8, Streptococcus pyogenes; 9, Streptococcus pneumoniae; 10, Streptococcus mitis; 11, Streptococcus salivarius; 12, Streptococcus sanguinis; 13, Streptococcus dysgalactiae; 14, Streptococcus gordonii; 15, Streptococcus sinensis; 16, Streptococcus constellatus; 17, Streptococcus anginosus; 18, Enterococcus faecium; 19, Enterococcus raffinosus; 20, Staphylococcus epidermidis; 21, Staphylococcus saprophyticus; 22, Lactobacillus jensenii; 23, Micrococcus yunnanensis; 24, Pseudomonas aeruginosa; 25, klebsiella pneumoniae; 26, Escherichia coli; 27, Candida albicans; 28, Candida tropicalis
Using the MCDA assay with vaginal and rectal swabs
Two hundreds vaginal and rectal swabs were tested by the MCDA assay, and the results of which were compared to that of enrichment/qPCR and CHROMagar culture. The results showed that S. agalactiae infection frequency detected by the MCDA assay was 8.0%, which was in accordance with enrichment/qPCR (Table 3). CHROMagar culture detected 6 samples positive, with the detection rate being 3.0% (Table 3). Each clinical specimen could be completed in 63.1 min from the beginning of the specimen collection until the interpretation of the MCDA assay, which significantly reduced the turn-around time. Thus, the MCDA assay was superior to enrichment/qPCR and CHROMagar culture in detecting S. agalactiae directly from vaginal and rectal swabs. The workflow of the S. agalactiae MCDA assay was displayed in Fig. 7.
Discussion
Since the universal screening of S. agalactiae was recommended for the women in the late stage of gestation, and the antibiotic was provided to those who tested positive for S. agalactiae. As a result, the incidence of neonatal infected with S. agalactiae has droped more than 60% (Carrillo-Avila et al. 2018). However, the traditional culture method could not absolutely effective identifying S. agalactiae. Then, the rapid and sensitive qPCR assays were introduced to detect S. agalactiae in recent years, and several genes frequently have been employed for the specific amplification of S. agalactiae, including cfb gene and sip gene(Cai et al. 2019; Zhao et al. 2019a, b). However, 3.8% of clinical isolates didn’t harbor cfb gene and 9.31% of of clinical isolates didn’t have sip gene, which may leading false-negative results (Phillips et al. 1980; Elbehiry et al. 2015). Moreover, cfb sequences of S. pyogenes, S. uberis and S. iniae have more than 70% sequence similarity to that of S. agalactiae, which may lead to false-positive results (Phillips et al. 1980). Thus, pcsB gene is alternative to cfb gene and sip gene for detecting S. agalactiae.
MCDA products were usually analyzed by turbidity meter, intercalating dyes, agarose gel electrophoresis and LFB (Wang et al. 2018; Gong et al. 2019; Li et al. 2020). Turbidity meter is an expensive device, which is not easy to obtain in the resource-limited hospitals, and the result is easily suffering from background interference. Although LFB is very portable, the lid of the MCDA reaction tube needed to be opened, increasing the chance of contamination. In this study, intercalating dyes and agarose gel electrophoresis were used to analyze the MCDA products. The results showed that there is no discrepancies on the analysis of the MCDA production between visual MG and 2% gel electrophoresis. However, MCDA products analyzed by 2% gel electrophoresis takes the other 20 min, and the result was obtained by the requirement of the gel imaging system, increasing the run cost. Not like gel electrophoresis, the results obtained by MG was at the end of amplification with naked eyes, eliminating the use of extra equipment and avoiding the risk of aerosol pollution. Thus, visual MG is more practical than 2% gel electrophoresis for analyzing the MCDA products.
This study presents a new isothermal amplification technique on the development of a MCDA diagnostic kit for rapid detection of S. agalactiae. The whole experiment only depends on a simple heater, with the weight of 1 kg, which is very easy to achieve in resource-limited restricts, not like an expensive thermal cycler that required for qPCR. The detection limit of the MCDA assay was 250 fg, which is more sensitive than 100 pg of PCR and 1 pg of LAMP (Pu et al. 2019). The MCDA assay depends on ten specific primers recognizing 10 different regions of pcsB sequence to accomplish amplification. Thus, MCDA in theory is more specific than those nucleic acid amplification methods of using less primer, such as qPCR of two primers and LAMP of six primers. Except for consideration of primers specificity from theoretical design, practical specificity analysis was also evaluated by 28 strains that no cross reactivity occured with non-S. agalactiae at 45 min. Thus, the MCDA assay is very portable, and exhibited excellent sensitivity and specificity.
Furthermore, direct detection from vaginal and rectal swabs was examined. It was found that 16 of 200 samples were positive for S. agalactiae by MCDA analysis, that being the same as that of enrichment/qPCR assay. This prevelence rate was in the range from 3.7 to 14.52% ever reported (Huang, et al. 2019). Here DNA extraction immediately from clinical samples by heat eliminated the requirement of a complex machine and tedious steps, greatly reducing labor cost and equipment cost. Moreover, the time to complete MCDA detection per clinical specimen was about 63.1 min, which eliminated enrichment culture required for qPCR, saving the time, especially for those at the time of delivery. The cost per test is US$ 4.9, including MCDA per reaction US$ 3.5 and the MG US$ 1.4, which is very economic. All these merits make it possible that the MCDA assay will be a valuable tool for point-of-care diagnosis of S. agalactiae.
In conclusion, we have successfully developed a MCDA technique that could immediately detect S. agalactiae from vaginal and rectal swabs. This visual method showed excellent sensitivity and specificity. The only device required is a basic heating equipment, which made this method simple for point-of-care test. Further validation with a number of clinical samples was required to evaluate the feasibility of this new developed method.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
References
Boyer KM, Gotoff SP (1985) Strategies for chemoprophylaxis of GBS early-onset infections. Antibiot Chemother (1971) 35:267–280
Cai Q, Fauvart M, Wiederkehr RS, Jones B, Cools P, Goos P, Vaneechoutte M, Stakenborg T (2019) Ultra-fast, sensitive and quantitative on-chip detection of group B streptococci in clinical samples. Talanta 192:220–225
Carrillo-Avila JA, Gutierrez-Fernandez J, Gonzalez-Espin AI, Garcia-Trivino E, Gimenez-Lirola LG (2018) Comparison of qPCR and culture methods for group B Streptococcus colonization detection in pregnant women: evaluation of a new qPCR assay. BMC Infect Dis 18(1):305
Connell TG, Rele M, Cowley D, Buttery JP, Curtis N (2007) How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics 119(5):891–896
Curry A, Bookless G, Donaldson K, Knowles SJ (2018) Evaluation of hibergene loop-mediated isothermal amplification assay for detection of group B streptococcus in recto-vaginal swabs: a prospective diagnostic accuracy study. Clin Microbiol Infect 24(10):1066–1069
Elbehiry A, Elsayed M, Marzouk E, Bathich Y, Alves D, Mario N (2015) Detection of virulence genes in Staphylococcus aureus and Streptococcus agalactiae isolated from mastitis in the Middle East. Microbiol Res 10:1–9
Feuerschuette O, Silveira SK, Cancelier A, Da SR, Trevisol DJ, Pereira JR (2018) Diagnostic yield of real-time polymerase chain reaction in the diagnosis of intrapartum maternal rectovaginal colonization by group B Streptococcus: a systematic review with meta-analysis. Diagn Microbiol Infect Dis 91(2):99–104
Gong L, Liu E, Che J, Li J, Liu X, Xu H, Liang J (2019) Multiple cross displacement amplification coupled with gold nanoparticles-based lateral flow biosensor for detection of the mobilized colistin resistance gene mcr-1. Front Cell Infect Microbiol 9:226
Hansen SM, Uldbjerg N, Kilian M, Sorensen UB (2004) Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J Clin Microbiol 42(1):83–89
Huang J, Lin XZ, Zhu Y, Chen C (2019) Epidemiology of group B streptococcal infection in pregnant women and diseased infants in mainland China. PediatrNeonatol 60(5):487–495
Kim SC, Kim S, Lee DH, Choi SR, Kim JS (2015) Effect of blood volume in standard anaerobic blood culture bottles of the BacT/ALERT 3D system used for the detection of pathogens and time to detection. PLoS ONE 10(2):e116728
Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA (2016) Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis 16(9):1076–1084
Li S, Jiang W, Huang J, Liu Y, Ren L, Zhuang L, Zheng Q, Wang M, Yang R, Zeng Y, Wang Y (2020) Highly sensitive and specific diagnosis of coronavirus disease 19 (COVID-19)by reverse transcription multiple cross displacement amplification-labelled nanoparticles biosensor. EurRespir J. https://doi.org/10.1183/13993003.02060-2020
Lin Y, Ye J, Luo M, Hu B, Wu D, Wen J, Yang C, Li Y, Ning Y (2019) Group B Streptococcus DNA copy numbers measured by digital PCR correlates with perinatal outcomes. Anal Chem 91(15):9466–9471
McGee L, Schrag SJ, Verani JR (2010) Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. Dept of Health and Human Services, Centers for Disease Control and Prevention, Atlanta
Phillips EA, Tapsall JW, Smith DD (1980) Rapid tube CAMP test for identification of Streptococcus agalactiae (Lancefield group B). J Clin Microbiol 12(2):135–137
Pu W, Wang Y, Yang N, Guo G, Li H, Li Q, Rehman NU, Zheng LX, Wang P, Han S, Zhou CC, Zheng JP, Zeng JF, Yuan J (2019) Investigation of Streptococcus agalactiae using pcsB-based LAMP in milk, tilapia and vaginal swabs in Haikou China. J Appl Microbiol 128(3):784–793
Rabaan AA, Saunar JV, Bazzi AM, Soriano JL (2017) Modified use of real-time PCR detection of group B Streptococcus in pregnancy. J Med Microbiol 66(10):1516–1520
Rosa-Fraile M, Spellerberg B (2017) Reliable detection of group B Streptococcus in the clinical laboratory. J Clin Microbiol 55(9):2590–2598
Shin JH, Pride DT (2019) Comparison of three nucleic acid amplification tests(NAATs) and culture for detection of group B Streptococcus (GBS) from enrichment broth. J Clin Microbiol 57(6):e01958-e2018
Tickler IA, Tenover FC, Dewell S, Le VM, Blackman RN, Goering RV, Rogers AM, Piwonka H, Jung-Hynes BD, Chen DJ, Loeffelholz MJ, Gnanashanmugam D, Baron EJ (2019) Streptococcus agalactiae strains with chromosomal deletions evade detection with molecular methods. J Clin Microbiol 57(4):e02040-e2118
Turner S, Pryer KM, Miao VP, Palmer JD (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46(4):327–338
Venkatesh M, Flores A, Luna RA, Versalovic J (2010) Molecular microbiological methods in the diagnosis of neonatal sepsis. Expert Rev Anti Infect Ther 8(9):1037–1048
Verani JR, McGee L (2010) SchragSJ (2010) Prevention of perinatal group B streptococcal disease—revised guidelines from CDC. MMWRRecomm Rep 59(10):1–36
Vieira LL, Perez AV, Machado MM, Kayser ML, Vettori DV, Alegretti AP, Ferreira CF, Vettorazzi J, Valerio EG (2019) Group B Streptococcus detection in pregnant women: comparison of qPCR assay, culture, and the XpertGBS rapid test. BMC Pregnancy Childbirth 19(1):532
Wang Y, Wang Y, Zhang L, Xu J, Ye C (2017) Visual and multiplex detection of nucleic acid sequence by multiple cross displacement amplification coupled with gold nanoparticle-based lateral flow biosensor. Sens Actuators B Chem 241:1283–1293
Wang Y, Yan W, Fu S, Hu S, Wang Y, Xu J, Ye C (2018) Multiple cross displacement amplification coupled with nanoparticles-based lateral flow biosensor for detection of Staphylococcus aureus and identification of methicillin-resistant S. aureus. Front Microbiol 9:907
Yancey MK, Schuchat A, Brown LK, Ventura VL, Markenson GR (1996) The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol 88(5):811–815
Zhao F, Niu L, Yan L, Nong J, Wang C, Wang J, Gao N, Zhu X, Wu L, Zheng F, Hu S (2019a) Establishment and application of multiple cross displacement amplification coupled with lateral flow biosensor (MCDA-LFB) for visual and rapid detection of Candida albicans in clinical samples. Front Cell Infect Microbiol 9:102
Zhao Y, Chen H, Liu H, Cai J, Meng L, Dong L, Zheng N, Wang J, Wang C (2019b) Quantitative polymerase chain reaction coupled with sodium dodecyl sulfate and propidium monoazide for detection of viable Streptococcus agalactiae in milk. Front Microbiol 10:661
Acknowledgements
Not applicable.
Funding
This work was supported by Bureau of Science and Technology of Shiyan City (grant number 19Y34) and Innovation Team of Hubei University of Medicine (grant number FDFR201801) and Hubei Province’s Outstanding Medical Academic Leader Program.
Author information
Authors and Affiliations
Contributions
XQC and JY designed the study. ZQD collected clinical specimens. DXL, YLG, FLC, and XBL conducted experiments. XQC wrote the manuscript. YJT and MFW were responsible for the overall supervision of the study and funded this study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study (number: 2020KS030) was approved by the Ethics Committee of Shiyan Taihe Hospital, and carried out in accordance with the declaration of Helsinki. All pregnant women who provided vaginal and rectal swabs signed on informed consent documents.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The other two sets of MCDA primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, X., Dou, Z., Yang, J. et al. Visual multiple cross displacement amplification for the rapid identification of S. agalactiae immediately from vaginal and rectal swabs. AMB Expr 11, 9 (2021). https://doi.org/10.1186/s13568-020-01168-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-020-01168-3