Comparison of Three Physical—Cognitive Training Programs in Healthy Older Adults: A Study Protocol for a Monocentric Randomized Trial
Abstract
:1. Introduction
Aims and Hypotheses
2. Materials and Methods
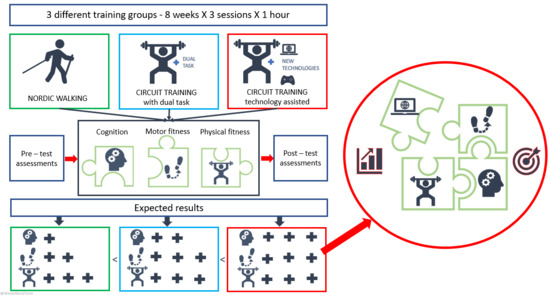
2.1. Study Design
2.2. Recruitment
2.3. Study Population and Eligible Criteria
2.4. Randomization
2.5. Informed Consent
2.6. Intervention Description
2.6.1. Nordic Walking (NW)
2.6.2. Conventional Circuit Training (CT-c)
2.6.3. Fitlight Trainer™ Circuit Training (CT-fit)
2.7. Data Collection and Outcome Measures
2.8. Cognitive Assessment
2.9. Motor Fitness Assessment
2.9.1. Balance
2.9.2. Functional Mobility
2.9.3. Flexibility
2.9.4. Motor Coordination
2.9.5. Gait Assessment
2.9.6. Dual-Task Assessment (DT)
2.10. Physical Fitness
2.10.1. Muscular Strength
2.10.2. Cardiovascular Capacities
2.11. Data Management
2.12. Sample Size
2.13. Statistical Analysis
3. Discussion
3.1. Differences in the Effectiveness of the Three Training Protocols on Cognitive, Physical, and Motor Capacities
3.2. Strength and Weakness of the Present Study
3.3. Benefits and Risk for Participants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.J.J.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008, 16, CD005381. [Google Scholar]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Kramer, A.F.; Colcombe, S. Fitness effects on the cognitive function of older adults: A meta-analytic study—Revisited. Perspect. Psychol. Sci. 2018, 13, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Viana, V.A.R.; Grassmann, V.; Dos Santos, R.V.T.; Santos, R.F.; Tufik, S.; De Mello, M.T. The impact of resistance exercise on the cognitive function of the elderly. Med. Sci. Sports Exerc. 2007, 39, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2017, 52, 154–160. [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. Aerobic-exercise and resistance-training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev. Cogn. Neurosci. 2019, 37, 100572. [Google Scholar] [CrossRef]
- Pesce, C. Shifting the focus from quantitative to qualitative exercise characteristics in exercise and cognition research. J. Sport Exerc. Psychol. 2012, 34, 766–786. [Google Scholar] [CrossRef] [Green Version]
- Voelcker-Rehage, C. Motor-skill learning in older adults—A review of studies on age-related differences. Eur. Rev. Aging Phys. Act. 2008, 5, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Physical and motor fitness are both related to cognition in old age. Eur. J. Neurosci. 2010, 31, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Niemann, C.; Godde, B.; Voelcker-Rehage, C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front. Aging Neurosci. 2014, 6, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Ludyga, S.; Gerber, M.; Pühse, U.; Looser, V.N.; Kamijo, K. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat. Hum. Behav. 2020, 4, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Baltes, P.B.; Lindenberger, U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol. Aging 1997, 12, 12–21. [Google Scholar] [CrossRef]
- Sleimen-Malkoun, R.; Temprado, J.-J.; Hong, S.L. Aging induced loss of complexity and dedifferentiation: Consequences for coordination dynamics within and between brain, muscular and behavioral levels. Front. Aging Neurosci. 2014, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, S.; Huxhold, O.; Lindenberger, U. Healthy mind in healthy body? A review of sensorimotor–cognitive interdependencies in old age. Eur. Rev. Aging Phys. Act. 2006, 3, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Sleimen-Malkoun, R.; Temprado, J.-J.; Berton, E. Age-related changes of movement patterns in discrete Fitts’ task. BMC Neurosci. 2013, 14, 145. [Google Scholar] [CrossRef] [Green Version]
- Heuninckx, S.; Wenderoth, N.; Debaere, F.; Peeters, R.; Swinnen, S.P. Neural basis of aging: The penetration of cognition into action control. J. Neurosci. 2005, 25, 6787–6796. [Google Scholar] [CrossRef]
- Heuninckx, S.; Wenderoth, N.; Swinnen, S.P. Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 2008, 28, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langeard, A.; Houdeib, R.; Saillant, K.; Kaushal, N.; Lussier, M.; Bherer, L. Switching ability mediates the age-related difference in timed up and go performance. J. Alzheimers Dis. 2019, 71, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Temprado, J.-J.; Torre, M.M.; Langeard, A.; Julien-Vintrou, M.; Devillers-Réolon, L.; Sleimen-Malkoun, R.; Berton, E. Intentional switching between bimanual coordination patterns in older adults: Is it mediated by inhibition processes? Front. Aging Neurosci. 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netz, Y. Is there a preferred mode of exercise for cognition enhancement in older age?—A narrative review. Front. Med. 2019, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, H.; Rezola-Pardo, C.; Echeverria, I.; Iturburu, M.; Gil, S.M.; Yanguas, J.J.; Irazusta, J.; Rodriguez-Larrad, A. Physical activity and fitness are associated with verbal memory, quality of life and depression among nursing home residents: Preliminary data of a randomized controlled trial. BMC Geriatr. 2018, 18, 80. [Google Scholar] [CrossRef] [Green Version]
- Berryman, C.; Stanton, T.R.; Bowering, K.J.; Tabor, A.; McFarlane, A.; Moseley, G.L. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin. Psychol. Rev. 2014, 34, 563–579. [Google Scholar] [CrossRef]
- Temprado, J.-J.; Julien-Vintrou, M.; Loddo, E.; Laurin, J.; Sleimen-Malkoun, R. Cognitive functioning enhancement in older adults: Is there an advantage of multicomponent training over nordic walking? Clin. Interv. Aging 2019, 14, 1503–1514. [Google Scholar] [CrossRef] [Green Version]
- Schiffer, T.; Knicker, A.; Hoffman, U.; Harwig, B.; Hollmann, W.; Strüder, H.K. Physiological responses to nordic walking, walking and jogging. Eur. J. Appl. Physiol. 2006, 98, 56–61. [Google Scholar] [CrossRef]
- Takeshima, N.; Islam, M.M.; Rogers, M.E.; Rogers, N.L.; Sengoku, N.; Koizumi, D.; Kitabayashi, Y.; Imai, A.; Naruse, A. Effects of nordic walking compared to conventional walking and band-based resistance exercise on fitness in older adults. J. Sports Sci. Med. 2013, 12, 422–430. [Google Scholar]
- Herold, F.; Hamacher, D.; Schega, L.; Müller, N.G. Thinking while moving or moving while thinking—Concepts of motor-cognitive training for cognitive performance enhancement. Front. Aging Neurosci. 2018, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Holtzer, R.; Verghese, J.; Xue, X.; Lipton, R.B. Cognitive processes related to gait velocity: Results from the Einstein aging study. Neuropsychology 2006, 20, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Wollesen, B.; Voelcker-Rehage, C. Training effects on motor–cognitive dual-task performance in older adults: A systematic review. Eur. Rev. Aging Phys. Act. 2014, 11, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Wollesen, B.; Scrivener, K.; Soles, K.; Billy, Y.; Leung, A.; Martin, F.; Iconomou, N.; McMahon, C.; Dean, C. Dual-task walking performance in older persons with hearing impairment: Implications for interventions from a preliminary observational study. Ear Hear. 2018, 39, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Alexander, G.E. Adaptive capacity: An evolutionary neuroscience model linking exercise, cognition, and brain health. Trends Neurosci. 2017, 40, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Zwierko, T.; Florkiewicz, B.; Fogtman, S.; Kszak-Krzyzanowska, A. The ability to maintain attention during visuomotor task performance in handball players and non-athletes. Cent. Eur. J. Sport Sci. Med. 2014, 7, 99–106. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.L.; Makrides, L.; Hitchcock, C.; Chypchar, T.; McCartney, N. Normal standards for an incremental progressive cycle ergometer test. Am. Rev. Respir. Dis. 1985, 131, 700–708. [Google Scholar]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Baldissera, F.; Cavallari, P.; Civaschi, P. Preferential coupling between voluntary movements of ipsilateral limbs. Neurosci. Lett. 1982, 34, 95–100. [Google Scholar] [CrossRef]
- Jeka, J.J.; Kelso, J.A.S. Manipulating symmetry in the coordination dynamics of human movement. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Heitger, M.H.; Goble, D.J.; Dhollander, T.; Dupont, P.; Caeyenberghs, K.; Leemans, A.; Sunaert, S.; Swinnen, S.P. Bimanual motor coordination in older adults is associated with increased functional brain connectivity—A graph-theoretical analysis. PLoS ONE 2013, 8, e62133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salesse, R.; Oullier, O.; Temprado, J.-J. Plane of motion mediates the coalition of constraints in rhythmic bimanual coordination. J. Mot. Behav. 2005, 37, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Temprado, J.-J.; Zanone, P.-G.; Monno, A.; Laurent, M. Attentional load associated with performing and stabilizing preferred bimanual patterns. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 1579–1594. [Google Scholar] [CrossRef]
- Temprado, J.-J.; Vercruysse, S.; Salesse, R.N.; Berton, E. A dynamic systems approach to the effects of aging on bimanual coordination. Gerontology 2010, 56, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Partington, J.E.; Leiter, R.G. Partington’s pathways test. Psychol. Serv. Cent. J. 1949, 1, 11–20. [Google Scholar]
- Osterrieth, P.A. The test of copying a complex figure: A contribution to the study of perception and memory. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Springer, B.A.; Marin, R.; Cyhan, T.; Roberts, H.; Gill, N.W. Normative Values for the Unipedal Stance Test with Eyes Open and Closed. J. Geriatr. Phys. Ther. 2007, 30, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Jones, C.J.; Rikli, R.E.; Max, J.; Noffal, G. The reliability and validity of a chair sit-and-reach test as a measure of hamstring flexibility in older adults. Res. Q. Exerc. Sport 1998, 69, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Dite, W.; Temple, V.A. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch. Phys. Med. Rehabil. 2002, 83, 1566–1571. [Google Scholar] [CrossRef]
- Ho, S.; Mohtadi, A.; Daud, K.; Leonards, U.; Handy, T.C. Using smartphone accelerometry to assess the relationship between cognitive load and gait dynamics during outdoor walking. Sci. Rep. 2019, 9, 3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastug, G.; Ozel-Kizil, E.T.; Sakarya, A.; Altintas, O.; Kirici, S.; Altunoz, U. Oral trail making task as a discriminative tool for different levels of cognitive impairment and normal aging. Arch. Clin. Neuropsychol. 2013, 28, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohannon, R.W. Hand-grip dynamometry predicts future outcomes in sging sdults. J. Geriatr. Phys. Ther. 2008, 31, 3–10. [Google Scholar] [CrossRef]
- Csuka, M.; Mccarty, D.J. Simple method for measurement of lower extremity muscle strength. Am. J. Med. 1985, 78, 77–81. [Google Scholar] [CrossRef]
- Woolf-May, K.; Meadows, S. Exploring adaptations to the modified shuttle walking test. BMJ Open 2013, 3, e002821. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef] [Green Version]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Kraft, E. Cognitive function, physical activity, and aging: Possible biological links and implications for multimodal interventions. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2012, 19, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Novick, J.J.; Bunting, M.F.; Dougherty, M.R.; Engle, R.W. Review of the evidence on, and fundamental questions about, efforts to improve executive functions, including working memory. In Cognitive and Working Memory Training: Perspectives from Psychology, Neuroscience, and Human Development; Diamond, A., Ling, D.S., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Participant ≥65 ≤80, good health condition, sedentary behavior Passed maximal cycle-ergometer effort test | Cognition < 26/30 on the MMSE IPAQ = highly active Sign of cognitive impairment Uncontrolled psychiatric or cardiovascular affections Uncorrected earing and/or visual impairment Psychotropic or bradicardizing medical treatments Participants familiar with the Fitlight Trainer™ methods |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, M.M.; Langeard, A.; Hugues, N.; Laurin, J.; Temprado, J.-J. Comparison of Three Physical—Cognitive Training Programs in Healthy Older Adults: A Study Protocol for a Monocentric Randomized Trial. Brain Sci. 2021, 11, 66. https://doi.org/10.3390/brainsci11010066
Torre MM, Langeard A, Hugues N, Laurin J, Temprado J-J. Comparison of Three Physical—Cognitive Training Programs in Healthy Older Adults: A Study Protocol for a Monocentric Randomized Trial. Brain Sciences. 2021; 11(1):66. https://doi.org/10.3390/brainsci11010066
Chicago/Turabian StyleTorre, Marta Maria, Antoine Langeard, Nicolas Hugues, Jérôme Laurin, and Jean-Jacques Temprado. 2021. "Comparison of Three Physical—Cognitive Training Programs in Healthy Older Adults: A Study Protocol for a Monocentric Randomized Trial" Brain Sciences 11, no. 1: 66. https://doi.org/10.3390/brainsci11010066