Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fruit Respiration Rate, Moisture Content and Colorimetry of Pulp and Seed
2.3. Pulp Mineral and Protein Content
2.4. Soluble Carbohydrates and Organic Acids
2.5. Total Phenols and Condensed Tannins
2.6. Analysis of Polyphenols by UHPLC-Q-Orbitrap HRMS
2.7. In Vitro Antioxidant Activity Assays
2.8. Statistical Analysis
3. Results and Discussion
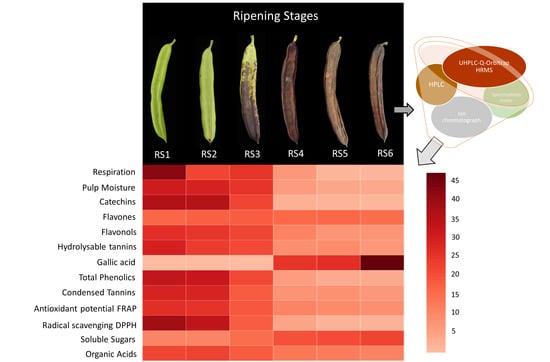
3.1. Critical Stages for Carob Harvest Maturity
3.2. Mineral and Protein Content
3.3. Soluble Carbohydrates and Organic Acids
3.4. Analysis of Polyphenols by UHPLC-Q-Orbitrap HRMS
3.5. Total Phenolics and Condensed Tannins
3.6. In Vitro Antioxidant Activity and Correlation with Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tous, J.; Romero, A.; Batlle, I. The Carob tree: Botany, horticulture, and genetic resources. Hortic. Rev. 2013, 41, 385–456. [Google Scholar]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Batlle, I.; Tous, J. Promoting the conservation and use of underutilized and neglected crops. In Carob Tree: Ceratonia siliqua L.; Bioversity International: Maccarese, Italy, 1997; p. 17. [Google Scholar]
- Bouzouita, N.; Khaldi, A.; Zgoulli, S.; Chebil, L.; Chekki, R.; Chaabouni, M.; Thonart, P. The analysis of crude and purified locust bean gum: A comparison of samples from different carob tree populations in Tunisia. Food Chem. 2007, 101, 1508–1515. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of Chemical Composition of Carob (Ceratonia siliqua): Protein, Fat, Carbohydrates, and Tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Zunft, H.; Lüder, W.; Harde, A.; Haber, B.; Graubaum, H.J.; Koebnick, C.; Grünwald, J. Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur. J. Nutr. 2003, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Theophilou, I.C.; Neophytou, C.M.; Constantinou, A.I. Carob and its Components in the Management of Gastrointestinal Disorders. J. Hepatol. Gastroenterol. 2017, 1, 5. [Google Scholar]
- Nasar-Abbas, S.M.; Huma, Z.; Vu, T.-H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Yousif, A.K.; Alghzawi, H. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Bosch, J.; Del Pino, F.G.; Ramoneda, J.; Retana, J. Fruiting phenology and fruit set of carob, Ceratonia siliqua L.(Cesalpinaceae). Isr. J. Plant Sci. 1996, 44, 359–368. [Google Scholar] [CrossRef]
- Von Haselberg, C.D. Vegetative Growth and Flower and Fruit Development in Carob Trees (Ceratonia siliqua L.) with Special Emphasis on Environmental Conditions at Marginal Production Sites in South Portugal; The University: Berlin, Germany, 2000. [Google Scholar]
- Morton, J.F. Fruits of Warm Climates; Julia, F., Ed.; Morton: Miami, FL, USA, 1987. [Google Scholar]
- Benchikh, Y.; Louaileche, H.; George, B.; Merlin, A. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind. Crops Prod. 2014, 60, 298–303. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Ydjedd, S.; Chaala, M.; Richard, G.; Kati, D.E.; López-Nicolás, R.; Fauconnier, M.-L.; Louaileche, H. Assessment of antioxidant potential of phenolic compounds fractions of Algerian Ceratonia siliqua L. pods during ripening stages. Int. Food Res. J. 2017, 24, 2041–2049. [Google Scholar]
- Vekiari, A.; Ouzounidou, G.; Gork, G.; Ozturk, M.; Asfi, M. Compositional changes of major chemical compounds in Greek carob pods during development. Bull. Chem. Soc. Ethiop. 2012, 26, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Othmen, K.B.; Elfalleh, W.; Lachiheb, B.; Haddad, M. Evolution of phytochemical and antioxidant activity of Tunisian carob (Ceratonia siliqua L.) pods during maturation. EuroBiotech J. 2019, 3, 135–142. [Google Scholar] [CrossRef]
- Kyratzis, A.; Antoniou, C.; Papayiannis, L.C.; Graziani, G.; Rouphael, Y.; Kyriacou, M.C. Pod morphology, primary and secondary metabolite profiles in non-grafted and grafted carob germplasm are configured by agro-environmental zone, genotype and growing season. Front. Plant Sci. 2020, in press. [Google Scholar]
- Kyriacou, M.C.; Emmanouilidou, M.G.; Soteriou, G.A. Asynchronous ripening behavior of cactus pear (Opuntia ficus-indica) cultivars with respect to physicochemical and physiological attributes. Food Chem. 2016, 211, 598–607. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Bremner, J. Total nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1149–1178. [Google Scholar]
- Antoniou, C.; Kyratzis, A.; Rouphael, Y.; Stylianou, S.; Kyriacou, M. Heat-and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material. Foods 2020, 9, 1364. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Sepperer, T.; Hernandez-Ramos, F.; Labidi, J.; Oostingh, G.J.; Bogner, B.; Petutschnigg, A.; Tondi, G. Purification of industrial tannin extract through simple solid-liquid extractions. Ind. Crop. Prod. 2019, 139, 111502. [Google Scholar] [CrossRef]
- Rached, I.; Barros, L.; Fernandes, I.P.; Santos-Buelga, C.; Rodrigues, A.E.; Ferchichi, A.; Barreiro, M.F.; Ferreira, I.C. Ceratonia siliqua L. hydroethanolic extract obtained by ultrasonication: Antioxidant activity, phenolic compounds profile and effects in yogurts functionalized with their free and microencapsulated forms. Food Fun. 2016, 7, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriacou, M.C.; Ioannidou, S.; Nikoloudakis, N.; Seraphides, N.; Papayiannis, L.C.; Kyratzis, A.C. Physicochemical characterization and trait stability in a genetically diverse ex situ collection of pomegranate (Punica granatum L.) germplasm from Cyprus. Sci. Hortic. 2020, 263, 109116. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- McGuire, R.G.J.H. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Rupasinghe, H.V. The role of polyphenols in quality, postharvest handling, and processing of fruits. In Postharvest Biology and Technology of Fruits, Vegetables and Flowers; Wiley-Blackwell Publishing: Ames, IA, USA, 2008. [Google Scholar]
- Özcan, M.M.; Arslan, D.; Gökçalik, H. Some compositional properties and mineral contents of carob (Ceratonia siliqua) fruit, flour and syrup. Int. J. Food Sci. Nutr. 2007, 58, 652–658. [Google Scholar]
- Kokkinofta, R.; Yiannopoulos, S.; Stylianou, M.A.; Agapiou, A. Use of Chemometrics for Correlating Carobs Nutritional Compositional Values with Geographic Origin. Metabolites 2020, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A. Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002. [Google Scholar]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Levander, O.A. Fruit and vegetable contributions to dietary mineral intake in human health and disease. HortScience 1990, 25, 1486–1488. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Food Database. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/362759/nutrients (accessed on 2 November 2020).
- Simsek, S.; Ozcan, M.M.; Al Juhaimi, F.; ElBabiker, E.; Ghafoor, K. Amino Acid and Sugar Contents of Wild and Cultivated Carob (Ceratonia siliqua) Pods Collected in Different Harvest Periods. Chem. Nat. Compd. 2017, 53, 1008–1009. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crops Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Alañón, M.E.; Oliver-Simancas, R.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Evolution of bioactive compounds of three mango cultivars (Mangifera indica L.) at different maturation stages analyzed by HPLC-DAD-q-TOF-MS. Food Res. Int. 2019, 125, 108526. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Yang, B.; Liu, P. Composition and Biological Activities of Hydrolyzable Tannins of Fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef]
- Lestario, L.N.; Howard, L.R.; Brownmiller, C.; Stebbins, N.B.; Liyanage, R.; Lay, J.O. Changes in polyphenolics during maturation of Java plum (Syzygium cumini Lam.). Food Res. Int. 2017, 100, 385–391. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Josuttis, M.; Verrall, S.; Stewart, D.; Krüger, E.; McDougall, G.J. Genetic and Environmental Effects on Tannin Composition in Strawberry (Fragaria × ananassa) Cultivars Grown in Different European Locations. J. Agric. Food Chem. 2013, 61, 790–800. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Custodio, L.; Fernandes, E.; Escapa, A.L.; Fajardo, A.; Aligue, R.; Albericio, F.; Neng, N.R.; Nogueira, J.M.; Romano, A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J. Agric. Food Chem. 2011, 59, 7005–7012. [Google Scholar] [CrossRef] [PubMed]
- Sebai, H.; Souli, A.; Chehimi, L.; Rtibi, K.; Amri, M.; El-Benna, J.; Sakly, M. In vitro and in vivo antioxidant properties of Tunisian carob (Ceratonia siliqua L.). J. Med. Plants Res. 2013, 7, 85–90. [Google Scholar]
- Kaneria, M.J.; Bapodara, M.B.; Chanda, S.V. Effect of Extraction Techniques and Solvents on Antioxidant Activity of Pomegranate (Punica granatum L.) Leaf and Stem. Food Anal. Methods 2012, 5, 396–404. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, gallotannins and their metabolites-the contribution to the anti-inflammatory effect of food products and medicinal plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]



| Source of Variance | Respiration | Moisture | L*—Pulp | L*—Seed | ||||
|---|---|---|---|---|---|---|---|---|
| PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | |
| Maturity (M) | 96.30 | *** | 99.07 | *** | 43.30 | *** | 71.15 | *** |
| Location (L) | 1.39 | *** | 0.08 | ** | 35.50 | *** | 13.29 | *** |
| Genotype (G) | 0.18 | ** | 0.05 | ns | 0.07 | ns | 0.09 | ns |
| M × L | 0.68 | *** | 0.12 | * | 8.74 | *** | 5.31 | *** |
| M × G | 0.41 | ** | 0.13 | ns | 3.30 | *** | 0.37 | ns |
| L × G | 0.06 | ns | 0.00 | ns | 0.27 | ns | 0.28 | * |
| M × L × G | 0.21 | * | 0.09 | * | 4.65 | *** | 0.32 | ns |
| Error | 0.77 | ns | 0.45 | ns | 4.17 | ns | 9.19 | ns |
| Means | ||||||||
| Maturity | (ml CO2 kg−1 h−1) | (%) | (0–100) | (0–100) | ||||
| RS1 | 47.86 ± 1.27 a | 75.83 ± 0.59 a | 80.17 ± 0.84 a | 55.91 ± 0.98 b | ||||
| RS2 | 24.64 ± 1.21 c | 72.33 ± 0.57 b | 79.99 ± 0.64 a | 68.23 ± 1.38 a | ||||
| RS3 | 28.10 ± 1.25 b | 62.58 ± 0.69 c | 76.52 ± 1.15 b | 55.02 ± 1.76 b | ||||
| RS4 | 7.68 ± 1.12 d | 14.83 ± 1.60 d | 74.40 ± 1.26 c | 39.49 ± 0.57 c | ||||
| RS5 | 1.33 ± 0.16 e | 9.75 ± 0.91 e | 74.12 ± 1.06 c | 35.95 ± 0.71 d | ||||
| RS6 | 1.17 ± 0.23 e | 8.58 ± 0.29 e | 70.68 ± 1.72 d | 35.38 ± 0.98 d | ||||
| Location | ||||||||
| Kalavasos (15 m) | 20.49 ± 3.00 a | 41.53 ± 4.99 a | 72.93 ± 0.90 | 41.51 ± 1.06 b | ||||
| Vavla (510 m) | 16.44 ± 2.76 b | 39.78 ± 5.17 b | 79.03 ± 0.41 | 52.99 ± 1.33 a | ||||
| Genotype | ||||||||
| Lefkaritiki | 17.73 ± 2.76 b | 41.31 ± 5.01 a | 76.12 ± 0.82 | 47.59 ± 1.41 | ||||
| Mavroteratsia | 19.20 ± 3.03 a | 40.00 ± 5.15 b | 75.84 ± 0.91 | 47.33 ± 1.26 | ||||
| Source of Variance | K | Mg | Ca | P | Protein | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | |
| Maturity (M) | 17.4 | *** | 52.2 | ** | 21.2 | *** | 26.4 | *** | 53.4 | *** |
| Location (L) | 2.0 | * | 1.8 | * | 26.3 | *** | 20.7 | *** | 33.5 | *** |
| Genotype (G) | 37.4 | *** | 10.8 | *** | 7.7 | ** | 9.0 | *** | 3.2 | *** |
| M × L | 7.4 | ** | 3.4 | ns | 11.3 | ** | 7.2 | *** | 2.8 | ** |
| M × G | 5.9 | ** | 6.0 | * | 7.4 | * | 5.6 | ** | 1.5 | * |
| L × G | 5.5 | ** | 0.3 | ns | 2.3 | * | 15.4 | *** | 0.6 | * |
| M × L × G | 8.1 | ** | 4.9 | ns | 3.0 | * | 5.4 | ** | 0.9 | ns |
| Error | 16.3 | 20.6 | 20.8 | 10.3 | 4.3 | |||||
| Means | ||||||||||
| Maturity | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (% dw) | |||||
| RS1 | 14.09 ± 072 ab | 1.60 ± 0.06 a | 2.32 ± 012 c | 0.59 ± 0.04 a | 7.11 ± 0.29 a | |||||
| RS2 | 14.46 ± 0.93 a | 1.43 ± 0.04 b | 2.95 ± 0.32 ab | 0.50 ± 0.07 b | 5.81 ± 0.39 b | |||||
| RS3 | 12.82 ± 066 bc | 1.42 ± 0.08 b | 3.47 ± 0.41 a | 0.4 0 ± 0.03 c | 4.92 ± 0.28 c | |||||
| RS4 | 13.62 ± 059 abc | 1.21 ± 0.07 c | 2.43 ± 022 bc | 0.36 ± 0.03 c | 4.14 ± 0.27 d | |||||
| RS5 | 12.58 ± 065 c | 1.14 ± 0.04 cd | 2.38 ± 0.16 bc | 0.39 ± 0.04 c | 4.59 ± 0.31 c | |||||
| RS6 | 11.22 ± 063 d | 1.02 ± 0.05 d | 2.21 ± 0.21 c | 0.37 ± 0.04 c | 4.21 ± 0.22 d | |||||
| Location | ||||||||||
| Kalavasos (15 m) | 13.50 ± 052 a | 1.27 ± 0.05 b | 2.13 ± 0.11 b | 0.51 ± 0.03 a | 5.96 ± 0.19 a | |||||
| Vavla (510 m) | 12.77 ± 033 b | 1.34 ± 0.04a | 3.12 ± 0.17 a | 0.36 ± 0.02 b | 4.30 ± 0.21 b | |||||
| Genotype | ||||||||||
| Lefkaritiki | 14.71 ± 040 a | 1.21 ± 0.04 b | 2.36 ± 0.15 b | 0.48 ± 0.03 a | 4.87 ± 0.23 b | |||||
| Mavroteratsia | 11.55 ± 028 b | 1.39 ± 0.05 a | 2.90 ± 0.16 a | 0.38 ± 0.02 b | 5.38 ± 0.25 a | |||||
| Source of Variance | Glucose | Fructose | Sucrose | Total Sugars | Malate | Citrate | Oxalate | Total Acids | Reducing/Sucrose | Sugars/Acids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | |
| Maturity (M) | 76.2 | *** | 8.0 | ns | 96.0 | *** | 91.7 | *** | 71.1 | *** | 50.6 | *** | 51.5 | *** | 72.1 | *** | 86.2 | *** | 78.9 | *** |
| Location (L) | 0.1 | ns | 3.9 | ns | 0.0 | ns | 0.1 | ns | 9.0 | *** | 0.7 | * | 14.4 | *** | 6.3 | *** | 0.5 | ** | 6.1 | *** |
| Genotype (G) | 1.1 | ns | 2.9 | ns | 0.5 | ** | 0.6 | * | 5.4 | *** | 9.4 | *** | 0.6 | ns | 6.3 | *** | 1.0 | *** | 2.9 | *** |
| M × L | 1.1 | ns | 5.9 | ns | 0.2 | ns | 0.3 | ns | 1.5 | ns | 16.1 | *** | 4.4 | * | 2.6 | * | 5.4 | *** | 4.3 | *** |
| M × G | 8.2 | *** | 16.4 | * | 0.3 | ns | 0.8 | ns | 2.5 | * | 1.6 | ns | 6.1 | ** | 2.3 | * | 2.6 | *** | 1.0 | ns |
| L × G | 1.0 | ns | 2.6 | ns | 0.0 | ns | 0.0 | ns | 0.0 | ns | 10.0 | *** | 3.1 | ** | 0.2 | ns | 0.1 | ns | 0.3 | ns |
| M × L × G | 0.9 | ns | 3.7 | ns | 0.7 | * | 0.7 | ns | 1.6 | ns | 3.1 | ** | 4.7 | * | 1.6 | ns | 2.0 | *** | 0.9 | ns |
| Error | 11.5 | 56.6 | 2.3 | 5.8 | 8.9 | 8.5 | 15.2 | 8.7 | 2.1 | 5.6 | ||||||||||
| Means | ||||||||||||||||||||
| Maturity | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (mg g−1 dw) | (Ratio) | (Ratio) | ||||||||||
| RS1 | 81.4 ± 3.8 b | 95.4 ± 3.0 | 56.6 ± 5.8 d | 233.4 ± 10.8 d | 12.7 ± 0.6 a | 2.5 ± 0.2 a | 0.8 ± 0.1 a | 16.0 ± 0.7 a | 3.5 ± 0.3 a | 15.1 ± 1.2 e | ||||||||||
| RS2 | 71.9 ± 2.0 b | 98.8 ± 2.1 | 64.3 ± 3.2 d | 235.0 ± 5.3 d | 13.1 ± 0.6 a | 2.7 ± 0.2 a | 0.6 ± 0.0 b | 16.4 ± 0.8 a | 2.7 ± 0.2 b | 14.7 ± 0.8 e | ||||||||||
| RS3 | 64.1 ± 2.3 c | 93.8 ± 3.7 | 176.9 ± 5.6 c | 334.8 ± 9.4 c | 10.9 ± 0.5 b | 1.8 ± 0.1 b | 0.6 ± 0.1 b | 13.3 ± 0.6 b | 0.9 ± 0.0 c | 25.7 ± 1.1 d | ||||||||||
| RS4 | 48.2 ± 1.7 d | 89.4 ± 3.0 | 310.4 ± 10.3 b | 448.0 ± 12.9 b | 7.6 ± 0.4 c | 1.4 ± 0.1 c | 0.4 ± 0.1 c | 9.4 ± 0.5 c | 0.5 ± 0.0 d | 49.3 ± 3.1 c | ||||||||||
| RS5 | 44.7 ± 1.4 d | 91.8 ± 3.2 | 327.6 ± 10.2 b | 464.1 ± 10.0 b | 7.0 ± 0.6 c | 1.5 ± 0.1 c | 0.3 ± 0.0 d | 8.7 ± 0.7 c | 0.4 ± 0.0 d | 57.1 ± 4.8 b | ||||||||||
| RS6 | 50.2 ± 1.7 d | 95.2 ± 2.9 | 353.2 ± 7.6 a | 498.7 ± 8.4 a | 6.3 ± 0.5 c | 1.3 ± 0.1 c | 0.3 ± 0.0 d | 7.8 ± 0.5 c | 0.4 ± 0.0 d | 67.3 ± 5.2 a | ||||||||||
| Location | ||||||||||||||||||||
| Kalavasos (15 m) | 59.7 ± 2.2 | 96.1 ± 1.6 | 216.2 ± 20.8 | 371.9 ± 19.0 | 8.6 ± 0.6 b | 1.9 ± 0.2 a | 0.4 ± 0.0 b | 10.9 ± 0.7 b | 1.3 ± 0.2 b | 43.9 ± 4.5 a | ||||||||||
| Vavla (510 m) | 60.5 ± 2.9 | 92.0 ± 1.9 | 213.6 ± 21.5 | 366.1 ± 19.0 | 10.6 ± 0.5 a | 1.8 ± 0.1 b | 0.6 ± 0.0 a | 13.0 ± 0.6 a | 1.5 ± 0.3 a | 32.5 ± 2.9 b | ||||||||||
| Genotype | ||||||||||||||||||||
| Lefkaritiki | 58.5 ± 1.8 b | 95.8 ± 1.8 | 206.3 ± 21.0 b | 360.5 ± 20.1 b | 10.4 ± 0.6 a | 2.1 ± 0.1 a | 0.5 ± 0.0 | 13.0 ± 0.7 a | 1.5 ± 0.3 a | 34.2 ± 3.5 b | ||||||||||
| Mavroteratsia | 61.7 ± 3.2 a | 92.3 ± 1.7 | 223.5 ± 21.2 a | 377.5 ± 17.8 a | 8.8 ± 0.5 b | 1.6 ± 0.1 b | 0.5 ± 0.0 | 10.9 ± 0.6 b | 1.3 ± 0.2 b | 42.2 ± 4.2 a | ||||||||||
| Compound | Theoretical Mass | Measured Mass | Accuracy (D ppm) | Tr (Min) |
|---|---|---|---|---|
| m/z (M-H)− | ||||
| gallic acid | 169.10143 | 169.101527 | 0.603 | 2.53 |
| methyl gallate | 183.02990 | 183.029770 | −0.710 | 6.72 |
| catechin | 289.07176 | 289.071790 | 0.104 | 7.23 |
| naringenin diglucoside | 595.16684 | 595.166900 | 0.101 | 7.75 |
| epicatechin | 289.07176 | 289.071130 | −2.179 | 7.94 |
| epigallocatechin gallate | 457.07763 | 457.077110 | −1.138 | 7.93 |
| gallocatechin gallate | 457.07763 | 457.077130 | −1.094 | 8.36 |
| vitexin | 431.09837 | 431.098130 | −0.557 | 9.07 |
| luteolin-7-O-glucoside | 447.09328 | 447.094100 | 1.834 | 9.44 |
| kaempferol-7-O-glucoside | 447.09328 | 447.093980 | 1.566 | 10.05 |
| isovitexin | 431.09837 | 431.098860 | 1.137 | 10.55 |
| gallocatechin | 305.06668 | 305.065000 | −5.507 | 10.87 |
| epigallocatechin | 305.06668 | 305.066790 | 0.361 | 7.44 |
| quercetin 3-O-glucoside | 463.08820 | 463.088340 | 0.302 | 9.6 |
| quercetin3-ramnoside | 447.09328 | 447.093560 | 0.626 | 10.22 |
| tetragalloyl glucose | 787.09994 | 787.098760 | −1.499 | 10.22 |
| trigalloyl glucose | 635.08899 | 635.088760 | −0.362 | 8.41 |
| digalloyl glucose | 483.07803 | 483.078650 | 1.283 | 7.66 |
| Source of Variance | Gallocatechin | Epigallocatechin gallate | Catechin | Gallocatechin gallate | Epigallocatechin | Epicatechin | Catechins | Naringenin diglucoside | Luteolin 7-O-glucoside | Vitexin | Isovitexin | Flavone glycosides | Kaempferol 3-O-glucoside | Quercetin 3-O-glucoside | Quercetin 3-ramnoside | Flavonol glycosides | Tetragalloyl glucose | Trigalloyl glucose | Digalloyl glucose | Hydrolyzable tannins | Gallic acid | Methyl gallate | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | |
| Maturity (M) | 59.3 | *** | 87.9 | *** | 70.3 | *** | 87.4 | *** | 75.4 | *** | 86.3 | *** | 83.7 | *** | 14.6 | *** | 34.2 | *** | 42.9 | *** | 50.2 | *** | 4.8 | *** | 59.0 | *** | 49.2 | *** | 55.9 | *** | 56.6 | *** | 85.4 | *** | 68.4 | *** | 49.6 | *** | 83.2 | *** | 78.8 | *** | 78.5 | *** |
| Location (L) | 1.0 | *** | 1.4 | *** | 6.5 | *** | 0.3 | ** | 3.6 | *** | 3.4 | *** | 0.2 | *** | 0.1 | 3.9 | *** | 0.4 | ** | 0.0 | 1.6 | ** | 0.2 | 0.7 | *** | 0.0 | 0.1 | 1.4 | *** | 5.4 | *** | 8.6 | *** | 2.8 | *** | 6.3 | *** | 5.0 | *** | |||||
| Genotype (G) | 1.0 | *** | 0.0 | 4.4 | *** | 0.3 | ** | 2.4 | *** | 0.1 | * | 0.8 | *** | 0.4 | 0.6 | * | 0.1 | 4.5 | *** | 2.7 | *** | 16.5 | *** | 9.9 | *** | 17.9 | *** | 15.8 | *** | 0.1 | 2.0 | *** | 0.2 | 0.3 | ** | 0.2 | ** | 0.4 | *** | |||||
| M × L | 15.1 | *** | 5.0 | *** | 6.3 | *** | 5.2 | *** | 3.4 | *** | 8.7 | *** | 3.9 | *** | 40.1 | *** | 19.4 | *** | 8.6 | *** | 29.9 | *** | 50.0 | *** | 4.1 | *** | 9.1 | *** | 6.6 | *** | 5.5 | *** | 3.4 | *** | 10.4 | *** | 9.4 | *** | 3.0 | *** | 7.0 | *** | 9.1 | *** |
| M × G | 7.7 | *** | 1.1 | *** | 10.8 | *** | 0.8 | ** | 8.7 | *** | 0.2 | ** | 3.5 | *** | 16.8 | *** | 20.8 | *** | 14.5 | *** | 3.8 | *** | 23.3 | *** | 6.9 | *** | 11.5 | *** | 5.5 | *** | 6.6 | *** | 0.8 | ** | 1.4 | *** | 8.3 | *** | 0.8 | *** | 3.9 | *** | 4.5 | *** |
| L × G | 6.9 | *** | 2.1 | *** | 0.1 | 2.0 | *** | 0.0 | 0.1 | ** | 3.6 | *** | 4.0 | *** | 4.2 | *** | 11.3 | *** | 3.3 | *** | 0.9 | ** | 8.3 | *** | 8.4 | *** | 8.0 | *** | 9.5 | *** | 2.7 | *** | 5.4 | *** | 5.7 | *** | 3.8 | *** | 0.1 | * | 0.6 | *** | ||
| M × L × G | 8.6 | *** | 1.9 | *** | 1.0 | *** | 2.7 | *** | 5.9 | *** | 0.5 | *** | 4.0 | *** | 17.5 | *** | 11.4 | *** | 19.5 | *** | 5.1 | *** | 11.9 | *** | 2.6 | *** | 10.4 | *** | 3.5 | *** | 4.8 | *** | 5.2 | *** | 5.7 | *** | 12.1 | *** | 5.2 | *** | 3.0 | *** | 1.0 | *** |
| Error | 0.4 | 0.7 | 0.8 | 1.4 | 0.5 | 0.6 | 0.3 | 6.6 | 5.7 | 2.7 | 3.3 | 4.9 | 2.5 | 0.8 | 2.6 | 1.2 | 1.1 | 1.3 | 6.1 | 0.9 | 0.7 | 1.0 | ||||||||||||||||||||||
| Maturity | Means (μg g−1 dw) | |||||||||||||||||||||||||||||||||||||||||||
| RS1 | 1070 ± 198 a | 554 ± 56 b | 600 ± 82 a | 312 ± 37 b | 9.9 ± 0.6 c | 3.9 ± 0.2 c | 2551 ± 239 a | 2.5 ± 0.5 c | 3.1 ± 0.6 b | 1.8 ± 0.5 c | 3.4 ± 0.5 a | 10.7 ± 1.8 bc | 80.4 ± 4.6 a | 57.2 ± 4.1 b | 52.7 ± 4.6 a | 190.3 ± 11.8 a | 575.7 ± 19.0 a | 204.7 ± 21.9 a | 85.8 ± 5.1 a | 866.2 ± 39.4 a | 0.0001 c | 0.0001 d | ||||||||||||||||||||||
| RS2 | 963 ± 143 b | 594 ± 18 a | 371 ± 39 c | 557 ± 31 a | 18.0 ± 2.4 a | 10.9 ± 1.1 a | 2514 ± 162 a | 3.4 ± 0.3 b | 2.7 ± 0.2 b | 2.5 ± 0.6 b | 2.9 ± 0.3 b | 11.5 ± 0.7 ab | 59.8 ± 5.6 c | 80.0 ± 16.1 a | 42.0 ± 4.3 b | 181.8 ± 25.1 a | 402.1 ± 20.1 b | 207.8 ± 9.2 a | 84.8 ± 3.6 a | 694.6 ± 29.8 b | 0.0001 c | 0.0001 d | ||||||||||||||||||||||
| RS3 | 565 ± 129 c | 318 ± 23 c | 414 ± 61 b | 219 ± 31 c | 13.7 ± 1.7 b | 6.8 ± 0.3 b | 1537 ± 213 b | 4.3 ± 0.4 a | 5.0 ± 0.5 a | 0.3 ± 0.1 d | 2.3 ± 0.3 c | 11.8 ± 0.6 a | 67.2 ± 9.8 b | 57.4 ± 8.7 b | 34.8 ± 5.3 c | 159.4 ± 22.4 b | 414.4 ± 47.1 b | 144.6 ± 13.8 b | 58.6 ± 2.3 b | 617.6 ± 61.1 c | 0.0001 c | 0.0001 d | ||||||||||||||||||||||
| RS4 | 32 ± 4 d | 57 ± 6 d | 39 ± 4 d | 11 ± 2 d | 0.6 ± 0.1 d | 0.5 ± 0.1 d | 140 ± 11 c | 3.2 ± 0.3 b | 2.7 ± 0.1 b | 3.0 ± 0.3 a | 1.5 ± 0.1 d | 10.4 ± 06 c | 35.3 ± 3.6 d | 15.5 ± 2.5 c | 25.6 ± 2.5 d | 76.4 ± 7.2 c | 106.3 ± 10.4 cd | 77.1 ± 6.1 c | 62.4 ± 4.6 b | 245.8 ± 19.9 d | 115.9 ± 14.8 b | 0.44 ± 0.06 c | ||||||||||||||||||||||
| RS5 | 7 ± 1 d | 55 ± 4 d | 32 ± 4 d | 5 ± 1 d | 0.3 ± 0.1d | 0.2 ± 0.0 d | 100 ± 8 c | 3.3 ± 0.4 b | 2.8 ± 0.2 b | 3.2 ± 0.2 a | 1.2 ± 0.2 e | 10.5 ± 0.7 c | 22.9 ± 5.0 e | 17.0 ± 3.2 c | 14.0 ± 3.4 e | 53.8 ± 11.5 d | 122.5 ± 12.9 c | 81.8 ± 8.5 c | 56.9 ± 4.6 b | 261.1 ± 22.2 d | 121.0 ± 17.3 b | 0.59 ± 0.07 a | ||||||||||||||||||||||
| RS6 | 7 ± 1 d | 33 ± 6 d | 17 ± 4 d | 2 ± 0 d | 0.2 ± 0.0 d | 0.2 ± 0.0 d | 64 ± 12 c | 3.6 ± 0.3 b | 2.8 ± 0.1 b | 2.3 ± 0.1 b | 1.1 ± 0.1 e | 9.8 ± 0.3 c | 22.5 ± 2.0 e | 14.8 ± 2.2 c | 14.6 ± 0.7 e | 51.9 ± 4.7 d | 91.0 ± 5.7 d | 63.0 ± 6.0 d | 47.5 ± 5.3 c | 201.5 ± 13.5 e | 215.5 ± 21.7 a | 0.51 ± 0.03 b | ||||||||||||||||||||||
| Location | ||||||||||||||||||||||||||||||||||||||||||||
| Kalavasos (15 m) | 500 ± 121 a | 240 ± 38 b | 176 ± 35 b | 172 ± 38 b | 5.5 ± 1.1 b | 2.9 ± 0.5 b | 1097 ± 217 b | 3.3 ± 0.3 | 2.9 ± 0.1 b | 2.1 ± 0.2 b | 2.1 ± 0.2 | 10.4 ± 0.5 b | 46.9 ± 5.2 | 43.5 ± 7.8 a | 30.9 ± 3.3 | 121.2 ± 15.5 | 261.1 ± 28.7 b | 113.1 ± 9.0 b | 60.0 ± 3.9 b | 434.2 ± 39.0 b | 98.6 ± 18.9 a | 0.32 ± 0.06 b | ||||||||||||||||||||||
| Vavla (510 m) | 382 ± 69 b | 299 ± 46 a | 315 ± 52 a | 196 ± 36 a | 8.7 ± 1.6 a | 4.5 ± 0.9 a | 1205 ± 189 a | 3.4 ± 0.2 | 3.5 ± 0.3 a | 2.3 ± 0.3 a | 2.0 ± 0.2 | 11.2 ± 0.6 a | 49.2 ± 4.6 | 37.2 ± 3.9 b | 30.4 ± 3.1 | 116.7 ± 10.9 | 309.5 ± 38.7 a | 146.6 ± 14.2 a | 72.0 ± 2.5 a | 528.1 ± 53.5 a | 52.2 ± 10.1 b | 0.19 ± 0.04 a | ||||||||||||||||||||||
| Genotype | ||||||||||||||||||||||||||||||||||||||||||||
| Lefkaritiki | 381 ± 74 b | 268 ± 44 | 189 ± 34 b | 196 ± 40 a | 5.8 ± 1.1 b | 3.6 ± 0.7 b | 1043 ± 180 b | 3.3 ± 0.2 | 3.1 ± 0.2 b | 2.1 ± 0.3 | 1.8 ± 0.2 b | 10.3 ± 0.4 b | 36.2 ± 4.0 b | 28.8 ± 3.8 b | 22.6 ± 2.3 b | 87.6 ± 9.5 b | 290.8 ± 36.4 | 139.9 ± 13.6 a | 65.2 ± 3.5 | 495.9 ± 50.6 a | 79.0 ± 14.8 a | 0.24 ± 0.04 b | ||||||||||||||||||||||
| Mavroteratsia | 500 ± 119 a | 271 ± 41 | 302 ± 54 a | 172 ± 33 b | 8.4 ± 1.6 a | 3.8 ± 0.7 a | 1259 ± 223 a | 3.5 ± 0.2 | 3.3 ± 0.3 a | 2.2 ± 0.2 | 2.3 ± 0.2 a | 11.3 ± 0.6 a | 59.9 ± 4.9 a | 51.9 ± 7.4 a | 38.6 ± 3.4 a | 150.3 ± 14.5 a | 279.9 ± 32.0 | 119.7 ± 10.5 b | 66.8 ± 3.4 | 466.4 ± 44.1 b | 71.8 ± 16.4 b | 0.28 ± 0.05 a | ||||||||||||||||||||||
| Source of Variance | Total Phenolics | Condensed Tannins | FRAP | DPPH | ||||
|---|---|---|---|---|---|---|---|---|
| PTV | Sig | PTV | Sig | PTV | Sig | PTV | Sig | |
| Maturity (M) | 98.0 | *** | 93.8 | *** | 95.8 | *** | 97.7 | *** |
| Location (L) | 0.2 | ** | 2.1 | *** | 1.0 | *** | 0.1 | ** |
| Genotype (G) | 0.1 | ** | 0.3 | *** | 0.3 | *** | 0.0 | ns |
| M × L | 0.3 | ** | 2.8 | *** | 1.5 | *** | 0.2 | ns |
| M × G | 0.5 | *** | 0.3 | *** | 0.2 | * | 0.1 | ns |
| L × G | 0.0 | ns | 0.1 | *** | 0.0 | ns | 0.3 | *** |
| M × L × G | 0.1 | ns | 0.4 | *** | 0.6 | *** | 0.9 | *** |
| Error | 0.7 | 0.2 | 0.7 | 0.7 | ||||
| Means | ||||||||
| Maturity | (mg g−1 dw) | (mg g−1 dw) | (μmol AAE g−1 dw) | (μmol AAE g−1 dw) | ||||
| RS1 | 181.1 ± 3.7 a | 198.3 ± 2.9 a | 1030 ± 11 a | 1712 ± 44 a | ||||
| RS2 | 172.9 ± 4.1 b | 196.8 ± 3.9 a | 989 ± 16 b | 1500 ± 56 b | ||||
| RS3 | 116.9 ± 3.5 c | 124.8 ± 3.2 b | 735 ± 11 c | 789 ± 12 c | ||||
| RS4 | 30.7 ± 2.8 d | 52.0 ± 3.2 c | 409 ± 15 d | 190 ± 21 d | ||||
| RS5 | 17.4 ± 2.0 e | 54.8 ± 6.3 c | 354 ± 28 e | 76 ± 10 e | ||||
| RS6 | 17.0 ± 1.3 e | 46.5 ± 8.5 d | 324 ± 25 f | 73.5 ± 7 e | ||||
| Location | ||||||||
| Kalavasos (15 m) | 86.1 ± 12.5 b | 102.4 ± 12.5 b | 610 ± 55 b | 702 ± 118 b | ||||
| Vavla (510 m) | 92.5 ± 11.6 a | 122.0 ± 10.1 a | 670 ± 45 a | 745 ± 111 a | ||||
| Genotype | ||||||||
| Lefkaritiki | 91.8 ± 12.4 a | 116.0 ± 11.4 a | 657 ± 51 a | 736 ± 116 | ||||
| Mavroteratsia | 86.8 ± 11.7 b | 108.4 ± 11.6 b | 624 ± 50 b | 711 ± 114 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriacou, M.C.; Antoniou, C.; Rouphael, Y.; Graziani, G.; Kyratzis, A. Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening. Antioxidants 2021, 10, 57. https://doi.org/10.3390/antiox10010057
Kyriacou MC, Antoniou C, Rouphael Y, Graziani G, Kyratzis A. Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening. Antioxidants. 2021; 10(1):57. https://doi.org/10.3390/antiox10010057
Chicago/Turabian StyleKyriacou, Marios C., Chrystalla Antoniou, Youssef Rouphael, Giulia Graziani, and Angelos Kyratzis. 2021. "Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening" Antioxidants 10, no. 1: 57. https://doi.org/10.3390/antiox10010057