Abstract
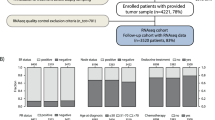
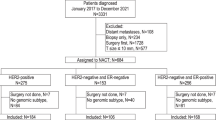
Analysis of breast cancer prognostic and predictive factors is still nowadays poorly accurate and standardized. The advent of multi-gene expression profiles (MGEPs) has improved the prediction of breast cancer outcome, particularly regarding early luminal breast cancers (LBCs). The availability in our Institute of EndoPredict® (EP), a last-generation prognostic gene signature assay, has prompted us to study a series of LBCs, firstly verifying its reproducibility on six routine representative cases, either presenting non-optimal preanalytical conditions or different tumor samples from the same patient; secondly, correlating EP results on 8 retrospectively recruited samples with patients’ follow-up; thirdly, applying prospectively EP on 100 routinely diagnosed cases, assessing the oncologists’ and pathologists’ attitude toward it. The complete reproducibility of EP on all the samples investigated in the first phase allowed to state that EP overcomes the detrimental effects of an inaccurate pre-analytic phase, determining the most appropriate prognostic and predictive parameters of breast cancer. The second phase confirmed EP as a fundamental tool in guiding therapeutic decision, improving the classical bio-pathological characterization and recovering 38% patients’ inadequately managed. Finally, the study disclosed how oncologists sometimes inadequately requested EP, but also how it allows a better stratification of breast cancer otherwise considered poorly aggressive and not requiring an EP test, such as G1 neoplasms or tubular histotype. In conclusion, the introduction of EP test in an Anatomic Pathology Department emerges as a useful tool in routine breast cancer diagnosis, both for the characterization of individual cases and, as a result, for more appropriate therapeutic choices.


Similar content being viewed by others
Data availability
The request for accessing the properly anonymized datasets analyzed in this article can be directed to Professor Angelo Sidoni: angelo.sidoni@unipg.it.
References
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical Oncology, College of American Pathologists (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72. https://doi.org/10.1043/1543-2165-134.7.e48
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical O, and College of American P American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. https://doi.org/10.1200/JCO.2006.09.2775
Khoury T (2018) Delay to formalin fixation (cold ischemia time) effect on breast cancer molecules. Am J Clin Pathol 149(4):275–292. https://doi.org/10.1093/ajcp/aqx164
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F (2015) Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 24(Suppl 2):S67–S72. https://doi.org/10.1016/j.breast.2015.07.017
Rakha EA, Starczynski J, Lee AH, Ellis IO (2014) The updated ASCO/CAP guideline recommendations for HER2 testing in the management of invasive breast cancer: a critical review of their implications for routine practice. Histopathology 64(5):609–615. https://doi.org/10.1111/his.12357
Singh K, Tantravahi U, Lomme MM, Pasquariello T, Steinhoff M, Sung CJ (2016) Updated 2013 College of American Pathologists/American Society of Clinical Oncology (CAP/ASCO) guideline recommendations for human epidermal growth factor receptor 2 (HER2) fluorescent in situ hybridization (FISH) testing increase HER2 positive and HER2 equivocal breast cancer cases; retrospective study of HER2 FISH results of 836 invasive breast cancers. Breast Cancer Res Treat 157(3):405–411. https://doi.org/10.1007/s10549-016-3824-x
Koren S, Bentires-Alj M (2015) Breast tumor heterogeneity: source of fitness, hurdle for therapy. Mol Cell 60(4):537–546. https://doi.org/10.1016/j.molcel.2015.10.031
Perou CM, Sorlie T, Eisen MB, Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel M (2011) Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol;22(8):1736–47. https://doi.org/10.1093/annonc/mdr304
Donovan MJ, Cordon-Cardo C (2017) Implementation of a precision pathology program focused on oncology-based prognostic and predictive outcomes. Mol Diagn Ther 21(2):115–123. https://doi.org/10.1007/s40291-016-0249-5
Kaul KL (2017) Preparing pathology for precision medicine: challenges and opportunities. Virchows Arch 471(2):141–146. https://doi.org/10.1007/s00428-017-2141-z
Compton CC, Robb JA, Anderson MW, Berry AB, Birdsong GG, Bloom KJ, Branton PA, Crothers JW, Cushman-Vokoun AM, Hicks DG, Khoury JD, Laser J, Marshall CB, Misialek MJ, Natale KE, Nowak JA, Olson D, Pfeifer JD, Schade A, Vance GH, Walk EE, Yohe SL (2019) Preanalytics and precision pathology: pathology practices to ensure molecular integrity of cancer patient biospecimens for precision medicine. Arch Pathol Lab Med 143(11):1346–1363. https://doi.org/10.5858/arpa.2019-0009-SA
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(8):1194–1220. https://doi.org/10.1093/annonc/mdz189
Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, Dietze O, Greil R, Jelen A, Sevelda P, Freibauer C, Muller V, Janicke F, Schmidt M, Kolbl H, Rody A, Kaufmann M, Schroth W, Brauch H, Schwab M, Fritz P, Weber KE, Feder IS, Hennig G, Kronenwett R, Gehrmann M, Gnant M, Investigators EP (2011) A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 17(18):6012–6020. https://doi.org/10.1158/1078-0432.CCR-11-0926
Denkert C, Kronenwett R, Schlake W, Bohmann K, Penzel R, Weber KE, Hofler H, Lehmann U, Schirmacher P, Specht K, Rudas M, Kreipe HH, Schraml P, Schlake G, Bago-Horvath Z, Tiecke F, Varga Z, Moch H, Schmidt M, Prinzler J, Kerjaschki D, Sinn BV, Muller BM, Filipits M, Petry C, Dietel M (2012) Decentral gene expression analysis for ER+/Her2- breast cancer: results of a proficiency testing program for the EndoPredict assay. Virchows Arch 460(3):251–259. https://doi.org/10.1007/s00428-012-1204-4
Kronenwett R, Bohmann K, Prinzler J, Sinn BV, Haufe F, Roth C, Averdick M, Ropers T, Windbergs C, Brase JC, Weber KE, Fisch K, Muller BM, Schmidt M, Filipits M, Dubsky P, Petry C, Dietel M, Denkert C (2012) Decentral gene expression analysis: analytical validation of the Endopredict genomic multianalyte breast cancer prognosis test. BMC Cancer 12:456. https://doi.org/10.1186/1471-2407-12-456
Muller BM, Keil E, Lehmann A, Winzer KJ, Richter-Ehrenstein C, Prinzler J, Bangemann N, Reles A, Stadie S, Schoenegg W, Eucker J, Schmidt M, Lippek F, Johrens K, Pahl S, Sinn BV, Budczies J, Dietel M, Denkert C (2013) The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PloS One 8(6):e68252. https://doi.org/10.1371/journal.pone.0068252
Muller BM, Brase JC, Haufe F, Weber KE, Budzies J, Petry C, Prinzler J, Kronenwett R, Dietel M, Denkert C (2012) Comparison of the RNA-based EndoPredict multigene test between core biopsies and corresponding surgical breast cancer sections. J Clin Pathol 65(7):660–662. https://doi.org/10.1136/jclinpath-2012-200716
EndoPredict®Instruction Manual. Sividon Diagnostics GmbHAs of: 26.02.2014
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2015) Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26(8):1533–1546. https://doi.org/10.1093/annonc/mdv221
Curigliano G, Burstein HJ, E PW, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thurlimann B, Panel Members of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast C (2019) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol;30(7):1181. https://doi.org/10.1093/annonc/mdy537
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, Committee EG (2015) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–v30. https://doi.org/10.1093/annonc/mdv298
Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, Gelmon K, Gentilini O, Harbeck N, Margulies A, Meirow D, Pruneri G, Senkus E, Spanic T, Sutliff M, Travado L, Peccatori F, Cardoso F (2017) ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast 35:203–217. https://doi.org/10.1016/j.breast.2017.07.017
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Nitz U, Gluz O, Christgen M, Kates RE, Clemens M, Malter W, Nuding B, Aktas B, Kuemmel S, Reimer T, Stefek A, Lorenz-Salehi F, Krabisch P, Just M, Augustin D, Liedtke C, Chao C, Shak S, Wuerstlein R, Kreipe HH, Harbeck N (2017) Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 165(3):573–583. https://doi.org/10.1007/s10549-017-4358-6
Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, Greil R, Dietze O, Luisser I, Klug E, Sedivy R, Bachner M, Mayr D, Schmidt M, Gehrmann MC, Petry C, Weber KE, Fisch K, Kronenwett R, Gnant M, Filipits M, Austrian Breast and Colorectal Cancer Study Group (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 109(12):2959–2964. https://doi.org/10.1038/bjc.2013.671
Fallowfield L, Matthews L, May S, Jenkins V, Bloomfield D (2018) Enhancing decision-making about adjuvant chemotherapy in early breast cancer following EndoPredict testing. Psycho-oncology 27(4):1264–1269. https://doi.org/10.1002/pon.4664
Author information
Authors and Affiliations
Contributions
All the authors have contributed to both conception, design of the study, acquisition, analysis, and interpretation of data, either drafting the article or revising it critically. All the authors have read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
Dr. Sidoni received speaker’s fees from Myriad Genetics. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Pelliccia, C., Caselli, E., Mandarano, M. et al. The implementation of a commercially available multi-gene profile test for breast cancer characterization in a department of pathology: what have we learned from the first 100 cases?. Virchows Arch 478, 1079–1087 (2021). https://doi.org/10.1007/s00428-020-02994-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02994-3