Abstract
Purpose
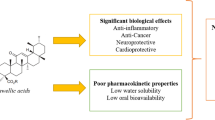
Macromolecules are important in polymer-based drug delivery systems as they help in specific targeting. This study explores the use of guggul gum (GG) and chitosan (Ch) in encapsulating boswellic acid (BA) which has high first-pass metabolism and low aqueous solubility for possible uses in inflammatory conditions.
Methods
Ionic complexation was used for nanoparticle formulation. The contents of guggul gum, chitosan, and boswellic acid were taken as independent variables, and their influence on encapsulation, particle size, and polydispersity were optimized by using Box-Behnken design. The developed formulation was assessed for drug release (in vitro) and release kinetics, stability, and preclinical pharmacokinetics. The formulation was also evaluated for inhibition of carrageenan-induced hind paw inflammation in rats.
Results
The nanoparticles were successfully prepared by ionic complexation method. The runs suggested by the design yielded a quadratic model for predicting the relationship between independent variables and responses. The optimum condition suggested by point prediction tool yielded spherical nanoparticles with 81.7% encapsulation, drug loading 21.2%, 377.19 nm size, and 0.201 PDI. The nanoparticles showed zero-order kinetics demonstrating an amalgamation of drug diffusion through matrix and erosion of polymeric medium.
Conclusion
Ionic complexation is a suitable method for nanopartile formulation. GG-Ch nanoparticles are beneficial for specific delivery and anti-inflammation of boswellic acid. The bioavailability of boswellic acid was increased by 11.38 times when given as a GG-Ch nanoparticle. The nanoparticles were stable at 25 °C for 6 months.









Similar content being viewed by others
References
Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82. https://doi.org/10.1038/nrd2614.
Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–35. https://doi.org/10.1038/nrd3499.
Worthington P, Langhans S, Pochan D. β-Hairpin peptide hydrogels for package delivery. Adv Drug Deliv Rev. 2017;110–111:127–36. https://doi.org/10.1016/j.addr.2017.02.002.
Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. https://doi.org/10.1126/science.aaf3627.
Long D, Gong T, Zhang Z, Ding R, Fu Y. Preparation and evaluation of a phospholipid-based injectable gel for the long term delivery of leuprolide acetate. Acta Pharm Sin B. 2016;6(4):329–35. https://doi.org/10.1016/j.apsb.2016.05.004.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–99. https://doi.org/10.2147/IJN.S68861.
Yue X, Dai Z. Recent advances in liposomal nanohybrid cerasomes as promising drug nanocarriers. Adv Colloid Interf Sci. 2014;207:32–42. https://doi.org/10.1016/j.cis.2013.11.014.
Guo S, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol Adv. 2014;32(4):778–88. https://doi.org/10.1016/j.biotechadv.2013.10.002.
Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8(1):34–50. https://doi.org/10.1016/j.apsb.2017.11.005.
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. https://doi.org/10.3389/fphar.2013.00177.
Tu J, Xu Y, Xu J, Ling Y, Cai Y. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in Caco-2 cells. Int J Biol Macromol. 2016;86:848–56. https://doi.org/10.1016/j.ijbiomac.2016.02.015.
Abul Kalam M, Khan AA, Khan S, Almalik A, Alshamsan A. Optimizing indomethacin-loaded chitosan nanoparticle size, encapsulation, and release using box–Behnken experimental design. Int J Biol Macromol. 2016;87:329–40. https://doi.org/10.1016/j.ijbiomac.2016.02.033.
Meng J, Sturgis TF, Youan B-BC. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. Eur J Pharm Sci. 2011;44(1):57–67. https://doi.org/10.1016/j.ejps.2011.06.007.
Ostanina ES, Varlamov VP, Yakovlev GI. Inhibition of lipase activity by low-molecular-weight chitosan. Appl Biochem Microbiol. 2007;43(6):655–60. https://doi.org/10.1134/S0003683807060154.
Sadreddini S, Safaralizadeh R, Baradaran B, Aghebati-Maleki L, Hosseinpour-Feizi MA, Shanehbandi D, et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol Lett. 2017;181:79–86. https://doi.org/10.1016/j.imlet.2016.11.013.
Zhu N, Rafi MM, DiPaola RS, Xin J, Chin C-K, Badmaev V, et al. Bioactive constituents from gum guggul (Commiphora wightii). Phytochemistry. 2001;56(7):723–7. https://doi.org/10.1016/S0031-9422(00)00485-4.
Sarup P, Bala S, Kamboj S. Pharmacology and phytochemistry of oleo-gum resin of Commiphora wightii (Guggulu). Scientifica (Cairo). 2015;2015:138039. https://doi.org/10.1155/2015/138039.
Gaur PK, Mishra S, Purohit S. Solid lipid nanoparticles of guggul lipid as drug carrier for transdermal drug delivery. Biomed Res Int. 2013;2013:750690. https://doi.org/10.1155/2013/750690.
Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. 2011;73(3):255–61. https://doi.org/10.4103/0250-474X.93507.
Sharma ML, Bani S, Singh GB. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int J Immunopharmacol. 1989;11(6):647–52. https://doi.org/10.1016/0192-0561(89)90150-1.
Dahmen U, Gu YL, Dirsch O, Fan LM, Li J, Shen K, et al. Boswellic acid, a potent antiinflammatory drug, inhibits rejection to the same extent as high dose steroids. Transplant Proc. 2001;33(1):539–41. https://doi.org/10.1016/S0041-1345(00)02131-X.
Ammon HPT. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006;72(12):1100–16. https://doi.org/10.1055/s-2006-947227.
Syrovets T, Büchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-Boswellic acids inhibit lipopolysaccharide-mediated TNF-α induction in monocytes by direct interaction with IκB kinases. J Immunol. 2005;174(1):498–506. https://doi.org/10.4049/jimmunol.174.1.498.
Sengupta K, Golakoti T, Marisetti A, Tummala T, Ravada S, Alluri K, et al. Inhibition of TNFα production and blocking of mitogen-activated protein kinase/NFκB activation in lipopolysaccharide-induced thp-1 human monocytes by 3-o-acetyl-11-keto-β-boswellic acid. J Food Lipids. 2009;16:325–44. https://doi.org/10.1111/j.1745-4522.2009.01150.x.
Skarke C, Kuczka K, Tausch L, Werz O, Rossmanith T, Barrett JS, et al. Increased bioavailability of 11-Keto-β-Boswellic acid following single oral dose frankincense extract administration after a standardized meal in healthy male volunteers: modeling and simulation considerations for evaluating drug exposures. J Clin Pharmacol. 2012;52(10):1592–600. https://doi.org/10.1177/0091270011422811.
Bagul P, Khomane KS, Bansal AK. Investigating permeability related hurdles in oral delivery of 11-keto-β-boswellic acid. Int J Pharm. 2014;464(1):104–10. https://doi.org/10.1016/j.ijpharm.2014.01.019.
Sterk V, Büchele B, Simmet T. Effect of food intake on the bioavailability of boswellic acids from a herbal preparation in healthy volunteers. Planta Med. 2004;70(12):1155–60. https://doi.org/10.1055/s-2004-835844.
Bairwa K, Jachak SM. Nanoparticle formulation of 11-keto-β-boswellic acid (KBA): anti-inflammatory activity and in vivo pharmacokinetics. Pharm Biol. 2016;54(12):2909–16. https://doi.org/10.1080/13880209.2016.1194437.
Bairwa K, Jachak SM. Development and optimisation of 3-acetyl-11-keto-β-boswellic acid loaded poly-lactic-co-glycolic acid-nanoparticles with enhanced oral bioavailability and in-vivo anti-inflammatory activity in rats. J Pharm Pharmacol. 2015;67(9):1188–97. https://doi.org/10.1111/jphp.12420.
Bernela M, Ahuja M, Thakur R. Enhancement of anti-inflammatory activity of glycyrrhizic acid by encapsulation in chitosan-katira gum nanoparticles. Eur J Pharm Biopharm. 2016;105:141–7. https://doi.org/10.1016/j.ejpb.2016.06.003.
Gaur PK, Shanmugam SK. Box-Behnken design–directed optimization of Wickerhamomyces anomalus–mediated biotransformation process to enhance the flavonoid profile of Polyherbal extract. J Pharm Innov. 2020. https://doi.org/10.1007/s12247-020-09467-9.
Gaur PK, Mishra S, Bajpai M, Mishra A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: in vitro drug release and pharmacokinetics studies. Biomed Res Int. 2014;2014:363404. https://doi.org/10.1155/2014/363404.
Gaur PK, Bajpai M, Mishra S, Verma A. Development of ibuprofen nanoliposome for transdermal delivery: physical characterization, in vitro/in vivo studies, and anti-inflammatory activity. Artif Cells Nanomed Biotechnol. 2016;44(1):370–5. https://doi.org/10.3109/21691401.2014.953631.
Yadav M, Ahuja M. Preparation and evaluation of nanoparticles of gum cordia, an anionic polysaccharide for ophthalmic delivery. Carbohydr Polym. 2010;81(4):871–7. https://doi.org/10.1016/j.carbpol.2010.03.065.
Robinson NG. 16 - Complementary and alternative medicine for cancer: the good, the bad, and the dangerous. In: Withrow SJ, Vail DM, Page RL, editors. Withrow and MacEwen’s small animal clinical oncology. 5th ed. Saint Louis: W.B. Saunders; 2013. p. 280–92.
Siemoneit U, Koeberle A, Rossi A, Dehm F, Verhoff M, Reckel S, et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br J Pharmacol. 2011;162(1):147–62. https://doi.org/10.1111/j.1476-5381.2010.01020.x.
Poeckel D, Tausch L, Kather N, Jauch J, Werz O. Boswellic acids stimulate arachidonic acid release and 12-lipoxygenase activity in human platelets independent of Ca2+ and differentially interact with platelet-type 12-lipoxygenase. Mol Pharmacol. 2006;70(3):1071–8. https://doi.org/10.1124/mol.106.024836.
Cao H, Yu R, Choi Y, Ma Z-Z, Zhang H, Xiang W, et al. Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacol Res. 2010;61(6):519–24. https://doi.org/10.1016/j.phrs.2010.02.007.
Fehrenbacher JC, Vasko MR, Duarte DB. Models of inflammation: carrageenan- or complete Freund’s adjuvant (CFA)–induced edema and hypersensitivity in the rat. Curr Protoc Pharmacol. 2012;56(1):5.4.1–5.4. https://doi.org/10.1002/0471141755.ph0504s56.
Acknowledgments
The authors graciously acknowledge the management of I.T.S College of Pharmacy for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaur, P.K., Puri, D., Singh, A.P. et al. Optimization and Pharmacokinetic Study of Boswellic Acid–Loaded Chitosan-Guggul Gum Nanoparticles Using Box-Behnken Experimental Design. J Pharm Innov 17, 485–500 (2022). https://doi.org/10.1007/s12247-020-09527-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09527-0