Abstract
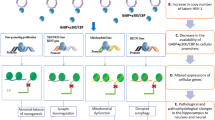
The cause of most Parkinson’s disease cases is unknown. However, it is well documented that mitochondrial dysfunction and misfolded α synuclein aggregation are important cellular abnormalities associated with the disease. In this paper, we use the microcompetition model to show how latent viruses, which infect the central and peripheral nervous systems, can cause the observed mitochondrial dysfunction and excess α synuclein aggregation, and eventually, Parkinson’s disease.
Similar content being viewed by others
References
Ahting U, Floss T, Uez N, Schneider-Lohmar I, Becker L, Kling E, Iuso A, Bender A, de Angelis MH, Gailus-Durner V, Fuchs H, Meitinger T, Wurst W, Prokisch H, Klopstock T (2009) Neurological phenotype and reduced lifespan in heterozygous Tim23 knockout mice, the first mouse model of defective mitochondrial import. BBA-Bioenergetics 1787(5):371–376
Bednar MM, Sturdevant CB, Tompkins LA, et al (2015) Compartmentalization, viral evolution, and viral latency of HIV in the CNS. Curr HIV/AIDS Rep. 12(2):262
Błaszczyk JW (2018) The emerging role of energy metabolism and neuroprotective strategies in Parkinson’s disease. Front Aging Neurosci 10:301
Blesa JR, Prieto-Ruiz JA, Hernández JM, Hernández-Yago J (2007) NRF-2 transcription factor is required for human TOMM20 gene expression. Gene 391(1–2):198–208
Brundin P, Melki R (2017) Prying into the prion hypothesis for Parkinson’s disease. The Journal of Neuroscience 37(41):9808–9818
Calaprice A (2000) The Expande Quotable Einstein. Princeton University Press P. 237
Chen C, Turnbull D, Reeve A (2019) Mitochondrial dysfunction in Parkinson’s disease—cause or consequence? Biology (Basel) 8(2):38
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. PNAS 110(19):E1817–E1826
Demishtein-Zohary K, Azem A (2016) The TIM23 mitochondrial protein import complex: function and dysfunction. Cell Tissue Res 367(1):33–41
Esteves AR, Arduíno DM, Silva DF, Oliveira CR, Cardoso SM (2011) Mitochondrial dysfunction: the road to alpha-synuclein oligomerization in PD. Parkinsons Dis 2011:693761
Franco-Iborra S, Cuadros T, Parent A, Romero-Gimenez J, Vila M, Perier C (2018) Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death and Disease 9(11):1122
Ganjam G, Bolte K, Matschke L, Neitemeier S, Dolga A, Höllerhage M, Höglinger G, Adamczyk A, Decher N, Oertel W, Culmsee C (2019) Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death and Disease 10(11):865
Grinde B (2013) Herpesviruses: latency and reactivation – viral strategies and host response. J Oral Microbiol 23:56
Grünewald A, Kumar KR, Sue CM (2019) New insights into the complex role of mitochondria in Parkinson’s disease. Prog Neurobiol 177:73
Hegarty, Shane & O Leary, Eimear & Solger, Franziska & Stanicka, Joanna & Sullivan, Aideen & O’Keeffe, Gerard. (2016). A small molecule activator of p300/CBP histone acetyltransferase promotes survival and neurite growth in a cellular model of Parkinson’s disease. Neurotox Res
Janknecht R (2002) The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol 17:657–668
Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P (2019) Triggers, facilitators, and aggravators: Redefining Parkinson’s disease pathogenesis. Trends Neurosci 42(1):4
Kulawiak B, Höpker J, Gebert M, Guiard B, Wiedemann N, Gebert N (2013) The mitochondrial protein import machinery has multiple connections to the respiratory chain. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1827(5): 612–616
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27
Limphaibool N, Iwanowski P, Holstad M, Kobylarek D and Kozubski WH (2019) Infectious etiologies of Parkinsonism: pathomechanisms and clinical implications. Front neurol 10:652
Lopes da Fonseca T, Villar-Piqué A, Outeiro TF (2015) The Interplay between alpha-synuclein clearance and spreading. Biomolecules 5(2):435
Lautenschläger J, Wagner-Valladolid S, Stephens AD, et al (2020) Intramitochondrial proteostasis is directly coupled to α-synuclein and amyloid β 1–42 pathologies [published online ahead of print, 2020 May 8]. J Biol Chem
Menon MB, Dhamija S (2018) Beclin 1 phosphorylation—at the Center of Autophagy Regulation. Front Cell Dev Biol 6:137
Metcalf DJ, García-Arencibia M, Hochfeld WE, Rubinsztein DC (2012) Autophagy and misfolded proteins in neurodegeneration. Exp Neurol 238(1):22–28
Moreno-Gonzalez I, Soto C (2011) Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol 22(5):482
Olsen L, Dowd E, McKernan D (2018) A role for viral infections in Parkinson’s etiology? Neuronal Signal 2(2)
Polansky H (2003) Microcompetition with foreign DNA and the origin of chronic disease. The Center for the Biology of Chronic Disease, New York
Polansky H, Javaherian A (2015) The latent cytomegalovirus decreases telomere length by microcompetition. Open Med (Wars) 10(1):294–296
Polansky H, Schwab H (2018) Copy number of latent viruses, oncogenicity, and the microcompetition model. Oncotarget 9(60):31568–31569
Polansky H, Schwab H (2018) How a disruption of the Competition between HIF-1 and p53 for limiting p300/CBP by Latent Viruses Can Cause Disease. Genes Cancer
Polansky H, Schwab H (2019) How latent viruses cause breast cancer: an explanation based on the microcompetition model. Bosn J Basic Med Sci 19(3):221–226
Popis M (2019) Dysfunction of mitochondria as the basis of Parkinson’s disease. Medical Journal of Cell Biology 6(4):174–181
Prieto-Ruiz JA, Alis R, García-Benlloch S, Sáez-Atiénzar S, Ventura I, Hernández-Andreu JM, Blesa JR (2018) Expression of the human TIMM23 and TIMM23B genes is regulated by the GABP transcription factor. BBA - Gene Regulatory Mechanisms 1861(2): 80–94
Ptaszyńska-Sarosiek I, Dunaj J, Zajkowska A et al (2019) Post-mortem detection of six human herpesviruses (HSV-1, HSV-2, VZV, EBV, CMV, HHV-6) in trigeminal and facial nerve ganglia by PCR. PeerJ 6:e6095
Rocha EM, De Miranda B, Sanders H (2017) Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 109:249–257
Shukla S, Tekwani BL (2020) Histone deacetylases inhibitors in neurodegenerative diseases. Neuroprotection and Neuronal Differentiation Front Pharmacol 11:537
Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R,1 Adame A, Wyss-Coray T, and Masliah E (2009) Beclin 1 Gene transfer activates autophagy and ameliorates the neurodegenerative pathology in α-synuclein models of Parkinson’s and Lewy body diseases. The Journal of Neuroscience 29(43) 13578 13588
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci 30(5):244–250
Surmeier DJ (2018) Determinants of dopaminergic neuron loss in Parkinson’s disease. The Febs Journal 285:3657–3668
Tulisiak C, Mercado G, Peelaerts W, Brundin L, Brundin P (2019) Can infections trigger alpha-synucleinopathies? Prog Mol Biol Transl Sci 168:299–322
Valor LM, Viosca J, Lopez-Atalaya JP, Barco A (2013) Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders. Curr Pharm Des 19(28):5051
Xue, Yongming & Wen, Hong & Shi, Xiaobing (2018) CBP/p300: Intramolecular and intermolecular regulations. Frontiers in Biology
Yu WH, Dorado B, Figueroa H, Wang L, Planel E, Cookson M, Clark L, Duff K (2009) Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric α-synuclein. Am J Pathol 175(2):736–747
Zhou CH, Zhang XP, Liu F, Wang W (2015) Modeling the interplay between the HIF-1 and p53 pathways in hypoxia. Sci Rep 5:13834
Zhu W, Swaminathan G, Plowey E (2014) GA binding protein augments autophagy via transcriptional activation of BECN1-PIK3C3 complex genes. Autophagy 10(9):1622–1636
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Polansky, H., Lori, G. How microcompetition with latent viruses can cause α synuclein aggregation, mitochondrial dysfunction, and eventually Parkinson’s disease. J. Neurovirol. 27, 52–57 (2021). https://doi.org/10.1007/s13365-020-00929-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-020-00929-x