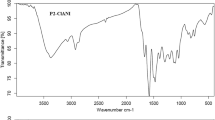
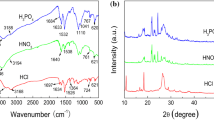
Abstract
Poly(o-bromophenol-co-N-methylaniline) [poly(OBP-co-NMA)] was electropolymerized in an acidic medium at 30°C under inert atmosphere. The formed polymer was characterized using IR spectroscopy, XRD, SEM and TGA analysis. The efficiency of the deposited polymer as a corrosion protection coating on a mild steel electrode in an acidic medium was investigated. Corrosion and impedance measurements reveal that the prepared polymer has excellent passivation properties. The free energy of adsorption of the prepared polymer on the electrode surface was in the range of ≈ − 19.5 kJ mol−1, which reveals a physical adsorption of the inhibitor molecules on the metallic surface.











Similar content being viewed by others
References
Arulepp, M, Permann, L, Leis, J, Perkson, A, Rumma, K, Jänes, A, Lust, E, “Influence of the Solvent Properties on the Characteristics of a Double Layer Capacitor.” J. Power Sources, 133 320–328 (2004). https://doi.org/10.1016/j.jpowsour.2004.03.026
Grennan, K, Killard, AJ, Hanson, CJ, Cafolla, AA, Smyth, MR, “Optimisation and Characterisation of Biosensors Based on Polyaniline.” Talanta, 68 1591–1600 (2006). https://doi.org/10.1016/j.talanta.2005.08.036
Karami, H, Mousavi, MF, Shamsipur, M, “A New Design for Dry Polyaniline Rechargeable Batteries.” J. Power Sources, 117 255–259 (2003). https://doi.org/10.1016/S0378-7753(03)00168-X
Saxena, V, Malhotra, B, “Prospects of Conducting Polymers in Molecular Electronics.” Curr. Appl. Phys., 3 293–305 (2003). https://doi.org/10.1016/S1567-1739(02)00217-1
Sengupta, PP, Barik, S, Adhikari, B, “Polyaniline as a Gas-Sensor Material.” Mater. Manuf. Process., 21 263–270 (2006). https://doi.org/10.1080/10426910500464602
Wang, Y, Jing, X, “Intrinsically Conducting Polymers for Electromagnetic Interference Shielding.” Polym. Adv. Technol., 16 344–351 (2005). https://doi.org/10.1002/pat.589
El-Lateef, HMABD, Elrouby, M, “Synergistic Inhibition Effect of Poly(Ethylene Glycol) and Cetyltrimethylammonium Bromide on Corrosion of Zn and Zn—Ni Alloys for Alkaline Batteries.” Trans. Nonferrous Met. Soc. China, 30 259–274 (2020). https://doi.org/10.1016/S1003-6326(19)65197-6
Fouda, A, Aldesoky, A, Elmorsi, M, Fayed, T, Atia, M, “New Eco-Friendly Corrosion Inhibitors Based on Phenolic Derivatives for Protection Mild Steel Corrosion.” Int. J. Electrochem. Sci., 8 10219–10238 (2013)
El-Lateef, HMA, Khalaf, MM, “Novel Dispersed Tl2O3-SiO2/Polyaniline Nanocomposites: In-Situ Polymerization, Characterization and Enforcement as a Corrosion Protective Layer for Carbon-Steel in Acidic Chloride Medium.” Colloids Surf. A Physicochem. Eng. Aspects, 573 95–111 (2019). https://doi.org/10.1016/j.colsurfa.2019.04.059
Li, Z, Hu, J, Li, Y, Zheng, F, Liu, J, “Self-Healing Active Anticorrosion Coatings with Polyaniline/Cerium Nitrate Hollow Microspheres.” Surf. Coat. Technol., 341 64–70 (2018). https://doi.org/10.1016/j.surfcoat.2017.11.054
Tammam, RH, Fekry, AM, Saleh, MM, “Understanding Different Inhibition Actions of Surfactants for Mild Steel Corrosion in Acid Solution.” Int. J. Electrochem. Sci, 11 1310–1326 (2016)
Wang, Y, “Preparation and Application of Polyaniline Nanofibers: An Overview.” Polym. Int., 67 650–669 (2018). https://doi.org/10.1002/pi.5562
Lakshmi, D, Rajendran, S, Sathiyabama, J, “Corrosion Inhibition by Phenols—An Overview.” Int. J. Nano-Corros. Sci. Eng., 3 1–18 (2016)
Sayyah, SM, El-Rabiei, MM, Abd El-Aafez, GM, Gaber, AF, “Electropolymerization of Ortho-Bromophenol on Pt-Electrode from Aqueous Acidic Solution; Kinetics, Mechanism, Electrochemical Studies and Characterization of the Polymer Obtained.” Int. J. Adv. Res., 3 65–84 (2015)
Aljeaban, N, Goni, L, Alharbi, B, Mazumder, J, Ali, S, Chen, T, Quraishi, M, Al-Muallem, HJ, “Polymers Decorated with Functional Motifs for Mitigation of Steel Corrosion: An Overview.” Int. J. Polym. Sci., 2020 (2020). https://doi.org/10.1155/2020/9512680
Veys-Renaux, D, Reguer, S, Bellot-Gurlet, L, Mirambet, F, Rocca, EJCS, “Conversion of Steel by Polyphenolic Model Molecules: Corrosion Inhibition Mechanism by Rutin, Esculin, Esculetol.” Corros. Sci., 136 1–8 (2018)
Li, Y, Zhang, H, Wang, X, Li, J, Wang, F, “Growth Kinetics of Oxide Films at the Polyaniline/Mild Steel Interface.” Corros. Sci., 53 4044–4049 (2011). https://doi.org/10.1016/j.corsci.2011.08.010
Shinde, VP, Patil, PP, “Investigation on Role of Monomer (s) During Electrochemical Polymerization of Aniline and Its Derivatives on Low Carbon Steel by XPS.” Electrochim. Acta, 78 483–494 (2012). https://doi.org/10.1016/j.electacta.2012.06.042
Abd El-Salam, H, Abd El-Hafez, G, Askalany, H, Fekry, A, “A Creation of Poly (N-2-Hydroxyethylaniline-co-2-Chloroaniline) for Corrosion Control of Mild Steel in Acidic Medium.” J. Bio-and Tribo-Corros., 6 1–14 (2020). https://doi.org/10.1007/s40735-020-00351-0
Bashir, S, Thakur, A, Lgaz, H, Chung, I-M, Kumar, A, “Computational and Experimental Studies on Phenylephrine as Anti-Corrosion Substance of Mild Steel in Acidic Medium.” J. Mol. Liq., 293 111539 (2019). https://doi.org/10.1016/j.molliq.2019.111539
El Ashry, ESH, El Nemr, A, Esawy, SA, Ragab, S, “Corrosion Inhibitors-Part II: Quantum Chemical Studies on the Corrosion Inhibitions of Steel in Acidic Medium by Some Triazole, Oxadiazole and Thiadiazole Derivatives.” Electroch. Acta, 51 3957–3968 (2006). https://doi.org/10.1016/j.electacta.2005.11.010
El-Salam, HMA, El-Hafez, GMA, Askalany, HG, Fekry, AM, “A Creation of Poly(N-2-Hydroxyethylaniline-co-2-Chloroaniline) for Corrosion Control of Mild Steel in Acidic Medium.” J. Bio- and Tribo-Corros., 6 53 (2020). https://doi.org/10.1007/s40735-020-00351-0
Khalaf, MM, Abd El-Lateef, HM, “Corrosion Protection of Mild Steel by Coating with TiO2 Thin Films Co-Doped with NiO and ZrO2 in Acidic Chloride Environments.” Mater. Chem. Phys., 177 250–265 (2016). https://doi.org/10.1016/j.matchemphys.2016.04.026
Abdrabo, WS, Elgendy, B, Soliman, KA, Abd El-Lateef, HM, Tantawy, AH, “Synthesis, Assessment and Corrosion Protection Investigations of Some Novel Peptidomimetic Cationic Surfactants: Empirical and Theoretical Insights.” J. Mol. Liq., 315 113672 (2020). https://doi.org/10.1016/j.molliq.2020.113672
Khalaf, MM, Tantawy, AH, Soliman, KA, Abd El-Lateef, HM, “Cationic Gemini-Surfactants Based on Waste Cooking Oil as New ‘Green’ Inhibitors for N80-Steel Corrosion in Sulphuric Acid: A Combined Empirical and Theoretical Approaches.” J. Mol. Struct., 1203 127442 (2020). https://doi.org/10.1016/j.molstruc.2019.127442
Aly, KI, Mohamed, MG, Younis, O, Mahross, MH, Abdel-Hakim, M, Sayed, MM, “Salicylaldehyde Azine-Functionalized Polybenzoxazine: Synthesis, Characterization, and Its Nanocomposites as Coatings for Inhibiting the Mild Steel Corrosion.” Prog. Org. Coat., 138 105385 (2020). https://doi.org/10.1016/j.porgcoat.2019.105385
Chafiq, M, Chaouiki, A, Lgaz, H, Salghi, R, Bhaskar, KV, Marzouki, R, Bhat, KS, Ali, IH, Khan, MI, Chung, I-M, “Inhibition Performances of Spirocyclopropane Derivatives for Mild Steel Protection in HCl.” Mater. Chem. Phys., 243 122582 (2020). https://doi.org/10.1016/j.matchemphys.2019.122582
Srivastava, V, Singh, MJJOAE, “Corrosion Inhibition of Mild Steel in Acidic Medium by Poly (Aniline-co-o-Toluidine) Doped with p-Toluene Sulphonic Acid.” J. Appl. Electrochem., 40 2135–2143 (2010)
Ashassi-Sorkhabi, H, Ghalebsaz-Jeddi, N, Hashemzadeh, F, Jahani, H, “Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Some Polyethylene Glycols.” Electrochim. Acta, 51 3848–3854 (2006). https://doi.org/10.1016/j.electacta.2005.11.002
Diggle, J, Downie, T, Goulding, C, “The Dissolution of Porous Oxide Films on Aluminium.” Electrochim. Acta, 15 1079–1093 (1970). https://doi.org/10.1016/0013-4686(70)85002-2
El-Rabiei, MM, Abd El-Aafez, GM, Gaber, AF, Farag, ZR, “Part I. Electropolymerization and Characterization of Polymer Coatings on Pt –Electrode.” Revista de Chimie, (in progress)
Kelen, T, Tüdos, F, “Analysis of the Linear Methods for Determining Copolymerization Reactivity Ratios. I. A New Improved Linear Graphic Method.” J. Macromol. Sci. A, 9 1–27 (1975)
Ismail, KM, El-Moneim, AA, Badawy, WA, “Stability of Sputter-Deposited Amorphous Mn-Ta Alloys in Chloride-Free and Chloride-Containing H2SO4 Solutions.” J. Electrochem. Soc., 148 C81–C87 (2001)
Huang, R, Han, Y, “The Effect of SMAT-Induced Grain Refinement and Dislocations on the Corrosion Behavior of Ti–25Nb–3Mo–3Zr–2Sn Alloy.” Mater. Sci. Eng. C, 33 2353–2359 (2013). https://doi.org/10.1016/j.msec.2013.01.068
Damaskin, BB, Petrii, OA, Batrakov, VV, Adsorption of Organic Compounds on Electrodes. Plenum, New York (1971)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abd El-Hafeez, G.M., El-Rabeie, M.M., Gaber, A.F. et al. Tailored polymer coatings as corrosion inhibitor for mild steel in acid medium. J Coat Technol Res 18, 581–590 (2021). https://doi.org/10.1007/s11998-020-00426-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-020-00426-0