Abstract
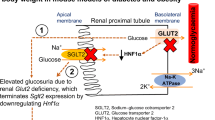
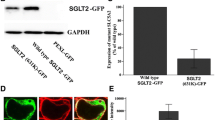
Inhibitors of sodium/glucose co-transporter 2 (SGLT2) are currently in clinical use for type 2 diabetes (T2D) treatment due to their anti-hyperglycemic effect exerted by the inhibition of glucose reabsorption in the kidney. Inhibition of SGLT2 is associated with improvement of renal outcomes in chronic kidney disease associated with T2D. Our study aimed to describe the renal-specific phenotypic consequences of the SGLT2-loss of function “Jimbee” mutation within the Slc5a2 mouse gene in a non-diabetic/non-obese background. The Jimbee mice displayed reduced body weight, glucosuria, polyuria, polydipsia, and hyperphagia but were normoglycemic, with no signs of baseline insulin resistance or renal dysfunction. Histomorphological analysis of the kidneys revealed a normal architecture and morphology of the renal cortex, but shrinkage of the glomerular and tubular apparatus, including Bowman’s space, glomerular tuft, mesangial matrix fraction, and proximal convoluted tubule (PCT). Immunofluorescent analysis of renal sections showed that SGLT2 was absent from the apical membrane of PCT of the Jimbee mice but remnant positive vesicles were detected within the cytosol or at the perinuclear interface. Renal localization and abundance of GLUT1, GLUT2, and SGLT1 were unchanged in the Jimbee genotype. Intriguingly, the mutation did not induce hepatic gluconeogenic gene expression in overnight fasted mice despite a high glucose excretion rate. The Jimbee phenotype is remarkably similar to humans with SLC5A2 mutations and provides a useful model for the study of SGLT2-loss of function effects on renal architecture and physiology, as well as for identifying possible novel roles for the kidneys in glucose homeostasis and metabolic reprogramming.









Similar content being viewed by others
References
Abdul-Ghani MA, DeFronzo RA, Norton L (2013) Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 62:3324–3328
Alatrach M, Laichuthai N, Martinez R, Agyin C, Ali AM, Al-Jobori H, Lavynenko O, Adams J, Triplitt C, DeFronzo R, Cersosimo E, Abdul-Ghani M (2020) Evidence against an important role of plasma insulin and glucagon concentrations in the increase in EGP caused by SGLT2 inhibitors. Diabetes 69:681–688
Atageldiyeva K, Fujita Y, Yanagimachi T, Mizumoto K, Takeda Y, Honjo J, Takiyama Y, Abiko A, Makino Y, Haneda M (2016) Sodium-glucose cotransporter 2 inhibitor and a low carbohydrate diet affect gluconeogenesis and glycogen content differently in the kidney and the liver of non-diabetic mice. PLoS ONE 11:e0157672
Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21:512–517
Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, Feder JN (2010) Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes therapy : research, treatment and education of diabetes and related disorders 1:57–92
Ferrannini E (2017) Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 26:27–38
Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124:499–508
Fishman B, Shlomai G, Twig G, Derazne E, Tenenbaum A, Fisman EZ, Leiba A, Grossman E (2019) Renal glucosuria is associated with lower body weight and lower rates of elevated systolic blood pressure: results of a nationwide cross-sectional study of 2.5 million adolescents. Cardiovascular Diabetology 18:124
Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, Harvie BM, Kinneally TL, Yeh SM, McCarthy DA, Koepsell H, Vallon V, Pollock C, Panchapakesan U, Forbes JM (2016) Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep 6:26428
Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, Hugo C (2014) The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 307:F317-325
Ghezzi C, Wright EM (2012) Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 303:C348-354
Hatanaka T, Ogawa D, Tachibana H, Eguchi J, Inoue T, Yamada H, Takei K, Makino H, Wada J (2016) Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose-induced oxidative stress in type 1 diabetic mice. Pharmacol Res Perspect 4:e00239
Huang WH, Chang CH, Huang CP, Wu HC, Hsieh IP (2017) The percentage of resected and ischemic volume determined by a geometric model is a significant predictor of renal functional change after partial nephrectomy. Int Braz J Urol 43:80–86
Hummel CS, Lu C, Loo DDF, Hirayama BA, Voss AA, Wright EM (2011) Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300:C14–C21
Jiang M, Wang Q, Karasawa T, Koo JW, Li H, Steyger PS (2014) Sodium-glucose transporter-2 (SGLT2; SLC5A2) enhances cellular uptake of aminoglycosides. PLoS ONE 9:e108941
Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, Moeckel GW, Samuel VT, Whaley JM, Shulman GI, Kibbey RG (2011) SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60:890–898
Kuhre RE, Ghiasi SM, Adriaenssens AE, Wewer Albrechtsen NJ, Andersen DB, Aivazidis A, Chen L, Mandrup-Poulsen T, Orskov C, Gribble FM, Reimann F, Wierup N, Tyrberg B, Holst JJ (2019) No direct effect of SGLT2 activity on glucagon secretion. Diabetologia 62:1011–1023
Li S, Yang Y, Huang L, Kong M, Yang Z (2019) A novel compound heterozygous mutation in SLC5A2 contributes to familial renal glucosuria in a Chinese family, and a review of the relevant literature. Mol Med Rep 19:4364–4376
Lupsa BC, Inzucchi SE (2018) Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia 61:2118–2125
Ly JP, Onay T, Sison K, Sivaskandarajah G, Sabbisetti V, Li L, Bonventre JV, Flenniken A, Paragas N, Barasch JM, Adamson SL, Osborne L, Rossant J, Schnermann J, Quaggin SE (2011) The Sweet Pee model for Sglt2 mutation. J Am Soc Nephrol 22:113–123
Maki T, Maeno S, Maeda Y, Yamato M, Sonoda N, Ogawa Y, Wakisaka M, Inoguchi T (2019) Amelioration of diabetic nephropathy by SGLT2 inhibitors independent of its glucose-lowering effect: a possible role of SGLT2 in mesangial cells. Scientific Reports 9:4703
Martinez R, Al-Jobori H, Ali AM, Adams J, Abdul-Ghani M, Triplitt C, DeFronzo RA, Cersosimo E (2018) Endogenous glucose production and hormonal changes in response to canagliflozin and liraglutide combination therapy. Diabetes 67:1182–1189
Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA (2014) Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124:509–514
Nagata T, Fukuzawa T, Takeda M, Fukazawa M, Mori T, Nihei T, Honda K, Suzuki Y, Kawabe Y (2013) Tofogliflozin, a novel sodium-glucose co-transporter 2 inhibitor, improves renal and pancreatic function in db/db mice. Br J Pharmacol 170:519–531
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657
Oemar BS, Byrd DJ, Brodehl J (1987) Complete absence of tubular glucose reabsorption: a new type of renal glucosuria (type 0). Clin Nephrol 27:156–160
Ohgaki R, Wei L, Yamada K, Hara T, Kuriyama C, Okuda S, Ueta K, Shiotani M, Nagamori S, Kanai Y (2016) Interaction of the sodium/glucose cotransporter (SGLT) 2 inhibitor canagliflozin with SGLT1 and SGLT2: inhibition kinetics, sidedness of action, and transporter-associated incorporation accounting for its pharmacodynamic and pharmacokinetic features. J Pharmacol Exp Ther 358:94–102
Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, Gerszten R, Patti M-E (2019) SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 4
Ottosson-Laakso E, Tuomi T, Forsen B, Gullstrom M, Groop PH, Groop L, Vikman P (2016) Influence of familial renal glycosuria due to mutations in the SLC5A2 gene on changes in glucose tolerance over time. PLoS ONE 11:e0146114
Packer M (2020) SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 43:508–511
Panayotova-Heiermann M, Loo DD, Kong CT, Lever JE, Wright EM (1996) Sugar binding to Na+/glucose cotransporters is determined by the carboxyl-terminal half of the protein. J Biol Chem 271:10029–10034
Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM (2012) Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55:2445–2455
Poulsen SB, Fenton RA, Rieg T (2015) Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24:463–469
Powell DR, DaCosta CM, Gay J, Ding ZM, Smith M, Greer J, Doree D, Jeter-Jones S, Mseeh F, Rodriguez LA, Harris A, Buhring L, Platt KA, Vogel P, Brommage R, Shadoan MK, Sands AT, Zambrowicz B (2013) Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab 304:E117-130
Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J (2005) Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54:3427–3434
Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V (2014) Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306:F188-193
Roder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H (2014) The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 9:e89977
Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H (2012) Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302:C1174-1188
Satoh H (2018) Pleiotropic effects of SGLT2 inhibitors beyond the effect on glycemic control. Diabetol Int 9:212–214
Scerbo D, Son NH, Sirwi A, Zeng L, Sas KM, Cifarelli V, Schoiswohl G, Huggins LA, Gumaste N, Hu Y, Pennathur S, Abumrad NA, Kershaw EE, Hussain MM, Susztak K, Goldberg IJ (2017) Kidney triglyceride accumulation in the fasted mouse is dependent upon serum free fatty acids. J Lipid Res 58:1132–1142
She P, Burgess SC, Shiota M, Flakoll P, Donahue EP, Malloy CR, Sherry AD, Magnuson MA (2003) Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes 52:1649–1654
Solini A, Sebastiani G, Nigi L, Santini E, Rossi C, Dotta F (2017) Dapagliflozin modulates glucagon secretion in an SGLT2-independent manner in murine alpha cells. Diabetes Metab 43:512–520
Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, Yamada M, Kitamura T (2019) SGLT1 in pancreatic alpha cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels. Mol Metab 19:1–12
Tang L, Wu Y, Tian M, Sjostrom CD, Johansson U, Peng XR, Smith DM, Huang Y (2017) Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab 313:E563-e576
Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, Eguchi J, Horiguchi CS, Nishii N, Yamada H, Takei K, Makino H (2014) Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 9:e100777
Thrailkill KM, Bunn RC, Uppuganti S, Ray P, Garrett K, Popescu I, Pennings JS, Fowlkes JL, Nyman JS (2020) Genetic ablation of SGLT2 function in mice impairs tissue mineral density but does not affect fracture resistance of bone. Bone 133:115254
Tomisato Wataru BK, Murray Anne, Beutler Bruce. Record for Jimbee, updated 2018–03–09. MUTAGENETIX (TM), B. Beutler and colleagues. Center for the Genetics of Host Defense, UT Southwestern, Dallas, TX. Accessed 2019–09–30. https://mutagenetix.utsouthwestern.edu
Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T (2011) SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22:104–112
Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T (2013) Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304:F156-167
Volker Vallon, Maria Gerasimova, Michael A. Rose, Takahiro Masuda, Joseph Satriano, Eric Mayoux, Hermann Koepsell, Scott C. Thomson, Timo Rieg, (2014) SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. American Journal of Physiology-Renal Physiology 306 (2):F194-F204
Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radovic N, Jadrijevic S, Aleksic I, Walles T, Sauvant C, Sabolic I, Koepsell H (2015) Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 467:1881–1898
Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, Levi M (2018a) The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents renal and liver disease in western diet induced obesity mice. Int J Mol Sci 19
Wang S, Zhao X, Zhang R, Wang C, Han Y, Shao L (2019) Identification of ten novel SLC5A2 mutations and determination of the renal threshold for glucose excretion in Chinese patients with familial renal glucosuria. Clin Chim Acta 490:102–106
Wang T, Bu CH, Hildebrand S, Jia G, Siggs OM, Lyon S, Pratt D, Scott L, Russell J, Ludwig S, Murray AR, Moresco EMY, Beutler B (2018b) Probability of phenotypically detectable protein damage by ENU-induced mutations in the Mutagenetix database. Nat Commun 9:441
Wang T, Zhan X, Bu CH, Lyon S, Pratt D, Hildebrand S, Choi JH, Zhang Z, Zeng M, Wang KW, Turer E, Chen Z, Zhang D, Yue T, Wang Y, Shi H, Wang J, Sun L, SoRelle J, McAlpine W, Hutchins N, Zhan X, Fina M, Gobert R, Quan J, Kreutzer M, Arnett S, Hawkins K, Leach A, Tate C, Daniel C, Reyna C, Prince L, Davis S, Purrington J, Bearden R, Weatherly J, White D, Russell J, Sun Q, Tang M, Li X, Scott L, Moresco EM, McInerney GM, Karlsson Hedestam GB, Xie Y, Beutler B (2015) Real-time resolution of point mutations that cause phenovariance in mice. Proc Natl Acad Sci U S A 112:E440-449
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375:323–334
Wright EM, Hirsch JR, Loo DD, Zampighi GA (1997) Regulation of Na+/glucose cotransporters. J Exp Biol 200:287–293
Wright EM, Loo DD, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91:733–794
Yu L, Lv JC, Zhou XJ, Zhu L, Hou P, Zhang H (2011) Abnormal expression and dysfunction of novel SGLT2 mutations identified in familial renal glucosuria patients. Hum Genet 129:335–344
Yu L, Xu Q, Hou P, Zhang H (2016) Decreased expression and function of sodium-glucose co-transporter 2 from a novel C-terminal mutation: a case report. BMC Nephrol 17:31
Acknowledgments
We thank Dr. Thomas Wilkop, from the Light Microscopy Core at the University of Kentucky, Lexington, KY, for imaging services and valuable advice regarding the SIM experiments. This research was supported by the Biospecimen Procurement & Translational Pathology Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558). The mouse strain used for this research project, C57BL/6J-Slc5a2m1Btlr/Mmmh, RRID: MMRRC_036517-MU, (the “Jimbee” strain) was obtained from the Mutant Mouse Resource and Research Center (MMRRC) at University of Missouri, an NIH-funded strain repository, and was donated to the MMRRC by Bruce Beutler, M.D., University of Texas Southwestern Medical Center.
Funding
This work was supported by grants from the National Institutes of Health, R21AR070620 (to K.M.T.) and R56DK084045 (to J.L.F.). Additional funding was provided by the University of Kentucky Barnstable Brown Diabetes Center Research Endowment. C.B.H and G.M.M were students enrolled in the HON385/ BIO395, respectively BIO395 Mentored Research Programs of the University of Kentucky under the mentorship of I.P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
Experiments on animals were approved by the local Institutional Animal Care and Use Committee (IACUC# 2015-2293) of the University of Kentucky, Lexington KY. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hughes, C.B., Mussman, G.M., Ray, P. et al. Impact of an SGLT2-loss of function mutation on renal architecture, histology, and glucose homeostasis. Cell Tissue Res 384, 527–543 (2021). https://doi.org/10.1007/s00441-020-03358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03358-8