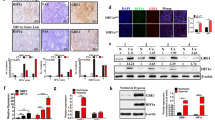
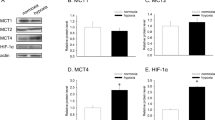
Abstract
Metabolic reprogramming leading to aerobic glycolysis, termed the “Warburg effect,” is a critical property of cancer cells. However, the precise mechanisms underlying this phenomenon are not fully understood. A growing body of evidence indicates that γ-glutamylcyclotransferase (GGCT), an enzyme involved in glutathione homeostasis that is highly expressed in many types of cancer, represents a promising therapeutic target. In this study, we identified GGCT as a novel regulator of hypoxia-inducible factor-1α (HIF-1α), a transcription factor that plays a role in hypoxia adaptation promoting aerobic glycolysis. In multiple human cancer cell lines, depletion of GGCT downregulated HIF-1α at the mRNA and protein levels. Conversely, in NIH3T3 mouse fibroblasts, overexpression of GGCT upregulated HIF-1α under normoxia. Moreover, depletion of GGCT downregulated HIF-1α downstream target genes involved in glycolysis, whereas overexpression of GGCT upregulated those genes. Metabolomic analysis revealed that modulation of GGCT expression induced a metabolic switch from the citric acid cycle to glycolysis under normoxia. In addition, we found that GGCT regulates expression of HIF-1α protein via the AMPK–mTORC1–4E-BP1 pathway in PC3 cells. Thus GGCT regulates the expression of HIF-1α in cancer cells, causing a switch to glycolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ngo DC, Ververis K, Tortorella SM, Karagiannis TC. Introduction to the molecular basis of cancer metabolism and the Warburg effect. Mol Biol Rep. 2015;42:819–23.
Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71.
Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11.
Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014;49:1–15.
Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–54.
Kim JW, Gao P, Dang CV. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007;26:291–8.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85.
Zeng L, Morinibu A, Kobayashi M, Zhu Y, Wang X, Goto Y, et al. Aberrant IDH3α expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene 2015;34:4758–66.
Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–53.
Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, et al. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaβ in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–7.
Yang XM, Wang YS, Zhang J, Li Y, Xu JF, Zhu J, et al. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:1873–9.
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83.
Kageyama S, Isono T, Iwaki H, Wakabayashi Y, Okada Y, Kontani K, et al. Identification by proteomic analysis of calreticulin as a marker for bladder cancer and evaluation of the diagnostic accuracy of its detection in urine. Clin Chem. 2004;50:857–66.
Kageyama S, Iwaki H, Inoue H, Isono T, Yuasa T, Nogawa M, et al. A novel tumor-related protein, C7orf24, identified by proteome differential display of bladder urothelial carcinoma. Proteomics Clin Appl. 2007;1:192–9.
Gromov P, Gromova I, Friis E, Timmermans-Wielenga V, Rank F, Simon R, et al. Proteomic profiling of mammary carcinomas identifies C7orf24, a gamma-glutamyl cyclotransferase, as a potential cancer biomarker. J Proteome Res. 2010;9:3941–53.
Li Y, Wu T, Wang Y, Yang L, Hu C, Chen L, et al. γ-Glutamyl cyclotransferase contributes to tumor progression in high grade serous ovarian cancer by regulating epithelial-mesenchymal transition via activating PI3K/AKT/mTOR pathway. Gynecol Oncol. 2018;149:163–72.
Jiang Z, Zhang C, Gan L, Jia Y, Xiong Y, Chen Y, et al. iTRAQ-based quantitative proteomics approach identifies novel diagnostic biomarkers that were essential for glutamine metabolism and redox homeostasis for gastric cancer. Proteomics Clin Appl. 2019;13:e1800038.
Uejima D, Nishijo K, Kajita Y, Ishibe T, Aoyama T, Kageyama S, et al. Involvement of cancer biomarker C7orf24 in the growth of human osteosarcoma. Anticancer Res. 2011;31:1297–305.
Takemura K, Kawachi H, Eishi Y, Kitagaki K, Negi M, Kobayashi M, et al. γ-Glutamylcyclotransferase as a novel immunohistochemical biomarker for the malignancy of esophageal squamous tumors. Hum Pathol. 2014;45:331–41.
Shen SH, Yu N, Liu XY, Tan GW, Wang ZX. Gamma-glutamylcyclotransferase promotes the growth of human glioma cells by activating Notch-Akt signaling. Biochem Biophys Res Commun. 2016;471:616–20.
Oakley AJ, Yamada T, Liu D, Coggan M, Clark AG, Board PG. The identification and structural characterization of C7orf24 as gamma-glutamyl cyclotransferase. An essential enzyme in the gamma-glutamyl cycle. J Biol Chem. 2008;283:22031–42.
Kageyama S, Hanada E, Ii H, Tomita K, Yoshiki T, Kawauchi A. Gamma-glutamylcyclotransferase: a novel target molecule for cancer diagnosis and treatment. Biomed Res Int. 2015;2015:345219.
Kageyama S, Ii H, Taniguchi K, Kubota S, Yoshida T, Isono T, et al. Mechanisms of tumor growth inhibition by depletion of γ-glutamylcyclotransferase (GGCT): a novel molecular target for anticancer therapy. Int J Mol Sci. 2018;19:E2054.
Hama S, Arata M, Nakamura I, Kasetani T, Itakura S, Tsuchiya H, et al. Prevention of tumor growth by needle-free jet injection of anti-C7orf24 siRNA. Cancer Gene Ther. 2012;19:553–7.
Ran R, Liu Y, Gao H, Kuang Q, Zhang Q, Tang J, et al. PEGylated hyaluronic acid-modified liposomal delivery system with anti-γ-glutamylcyclotransferase siRNA for drug-resistant MCF-7 breast cancer therapy. J Pharm Sci. 2015;104:476–84.
He Z, Wang S, Shao Y, Zhang J, Wu X, Chen Y, et al. Ras downstream effector GGCT alleviates oncogenic stress. iScience. 2019;19:256–66.
Ii H, Yoshiki T, Hoshiya N, Uenishi J. Synthesis and GGCT inhibitory activity of N-glutaryl-L-alanine analogues. Chem Pharm Bull. 2016;64:785–92.
Yoshiya T, Ii H, Tsuda S, Mochizuki M, Kageyama S, Yoshiki T. Design of fluorogenic probes and fluorescent-tagged inhibitors for γ-glutamyl cyclotransferase. J Pept Sci. 2017;23:618–23.
Ii H, Yoshiya T, Nakata S, Taniguchi K, Hidaka K, Tsuda S, et al. A novel prodrug of a γ-glutamylcyclotransferase inhibitor suppresses cancer cell proliferation in vitro and inhibits tumor growth in a xenograft mouse model of prostate cancer. ChemMedChem 2018;13:155–63.
Takagi H, Ii H, Kageyama S, Hanada E, Taniguchi K, Yoshiya T, et al. Blockade of γ-glutamylcyclotransferase enhances docetaxel growth inhibition of prostate cancer cells. Anticancer Res. 2019;39:4811–6.
Matsumura K, Nakata S, Taniguchi K, Ii H, Ashihara E, Kageyama S, et al. Depletion of γ-glutamylcyclotransferase inhibits breast cancer cell growth via cellular senescence induction mediated by CDK inhibitor upregulation. BMC Cancer. 2016;16:748.
Taniguchi K, Matsumura K, Ii H, Kageyama S, Ashihara E, Chano T, et al. Depletion of gamma-glutamylcyclotransferase in cancer cells induces autophagy followed by cellular senescence. Am J Cancer Res. 2018;8:650–61.
Taniguchi K, Matsumura K, Kageyama S, Ii H, Ashihara E, Chano T, et al. Prohibitin-2 is a novel regulator of p21WAF1/CIP1 induced by depletion of γ-glutamylcyclotransferase. Biochem Biophys Res Commun. 2018;496:218–24.
Taniguchi K, Ii H, Kageyama S, Takagi H, Chano T, Kawauchi A, et al. Depletion of gamma-glutamylcyclotransferase inhibits cancer cell growth by activating the AMPK-FOXO3a-p21 axis. Biochem Biophys Res Commun. 2019;517:238–43.
Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–14.
Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12.
Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003;144:5179–83.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26.
Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18.
Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–8.
Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64.
Devic S. Warburg effect - a consequence or the cause of carcinogenesis? J Cancer. 2016;7:817–22.
Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20:E238.
Doe MR, Ascano JM, Kaur M, Cole MD. Myc posttranscriptionally induces HIF1 protein and target gene expression in normal and cancer cells. Cancer Res. 2012;72:949–57.
Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D’Angeli L, Bartoli A, et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging. 2010;2:823–42.
TeSlaa T, Teitell MA. Techniques to monitor glycolysis. Methods Enzymol. 2014;542:91–114.
Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2015;34:2239–50.
Kim MH, Jeong YJ, Cho HJ, Hoe HS, Park KK, Park YY, et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol Rep. 2017;37:777–84.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science, grant numbers 20K07623 and 16K08722; the Ministry of Education, Culture, Sports, Science and Technology–Supported Program for the Strategic Research Foundation at Private Universities 2015–2019; AMED Grant Number JP19nk0101386; the Takeda Science Foundation; and the Kyoto Pharmaceutical University Fund for the Promotion of Collaborative Research.
Author information
Authors and Affiliations
Contributions
KT performed most of the experiments, analyzed the data, and wrote the manuscript. S Kageyama and S Kubota performed the metabolome analysis. CM, SA, and HI performed some experiments. S Kageyama, EA, MH, TS, and AK supervised and designed experiments. SN designed the study, supervised the research, and wrote the manuscript. All authors reviewed the manuscript and accepted the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Taniguchi, K., Kageyama, S., Moyama, C. et al. γ-Glutamylcyclotransferase, a novel regulator of HIF-1α expression, triggers aerobic glycolysis. Cancer Gene Ther 29, 37–48 (2022). https://doi.org/10.1038/s41417-020-00287-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00287-0
This article is cited by
-
Identification of c-Met as a novel target of γ-glutamylcyclotransferase
Scientific Reports (2023)